Meet our scientists!
 BackTopics Page
BackTopics Page
Inflammation is a critical immune response to attacks from bacteria, viruses, and other harmful substances. It functions to eliminate these harmful substances and clear out dead and damaged tissue. However, inflammation also has deleterious effects on the body. For example, neuroinflammation contributes to the severity of many neurodegenerative diseases. Thus, it is crucial to understand the molecular and cellular pathways causing inflammation. One of the goals of the Visual Research Project, led by Takayuki Harada, at TMIMS is to better understand diseases such as glaucoma or multiple sclerosis (MS) in order to develop more effective treatments. Previously, members from this project, including Xiaoli Guo, discovered that knocking out apoptosis signal-regulating kinase 1 (ASK1), a gene implicated in neuroinflammation, decreases the severity of a mouse model of MS. More recently, Guo and her colleagues identified the cell types and cellular and molecular interactions necessary for ASK1’s role in inducing neuroinflammation in MS. This work, “ASK1 signaling regulates phase-specific glial interactions during neuroinflammation,” was published in the Proceeding of the National Academy of Science, USA (Proc Natl Acad Sci U S A. 2022 Feb 8;119(6):e2103812119. doi: 10.1073/pnas.2103812119). We spoke to Dr. Guo about her work and her interest in science.

How did you become interested in science?
I was born in the countryside in China. We were very poor, so we had to be very self-sufficient. We grew our own crops and raised farm animals such as chickens and pigs for food. I think I naturally became interested in biology because I was exposed to animals and living things from such an early age.
Why did you decide to pursue research in Japan?
When I was a master’s student in China, I came to Japan as an exchange student between universities. I enjoyed it so much that I applied for a scholarship from the Japanese government and came to Japan for my Ph.D. I later pursued post-doctoral work in the United States, but I missed my husband in Japan, so I returned here after my post-doc and joined Dr. Harada’s lab at TMIMS, since I enjoy working on visual systems and neurodegenerative diseases.
Could you explain your latest publication on ASK1 and neuroinflammation?
In 2010, we published a paper on the role of ASK1 in experimental autoimmune encephalomyelitis (EAE), a mouse model for MS. MS is an inflammatory autoimmune disease characterized by demyelination of neurons. EAE is a classic model for MS that is generated by injecting a myelin basic protein peptide into mice to generate an autoimmune response to myelin. Similar to MS, EAE induces demyelination of neurons, severe neuroinflammation, and neuronal death. We found that knocking out ASK1 decreases EAE symptoms, but we didn’t know exactly why this happened. We didn’t know the cell types where ASK1 was activated, and we didn’t know the pathway through which ASK1 affects the severity of EAE. ASK1 is also involved in other neurodegenerative diseases including glaucoma, amyotrophic lateral sclerosis, Alzheimer’s disease, and Parkinson’s disease, so understanding how ASK1 induces inflammation should be important for developing treatments for many diseases.
In our current paper, we worked to understand the cells and pathways that are activated by ASK1 to increase disease symptoms. Various types of immune cells are known to be involved in neuroinflammation, including T-cells, dendritic cells, microglia, macrophages, and astrocytes. We made conditional knockouts of ASK1 in each of these cell types to understand which cells need to express ASK1 to induce neuroinflammation. Altogether we found that ASK1 is needed in microglia early during EAE to start the inflammatory process, and then it’s later needed in astrocytes to maintain inflammation. To start inflammation, many proinflammatory cytokine/chemokine signaling genes need to be expressed, and ASK1 in microglia is needed for this to occur. Activation of microglia in turn, activates astrocytes which are needed to maintain inflammation. We found that this interaction between microglia and astrocytes also requires ASK1 in both microglia and astrocytes. Without ASK1 in both cell types, activation of astrocytes doesn’t occur. Altogether, we identified different cell interactions and molecular interactions through which ASK1 causes and maintains neuroinflammation. We hope to use this information to devise future strategies for treating neurodegenerative diseases.
What are your future plans?
We’re interested in developing novel treatments for glaucoma and MS. To do this, we’re pursuing three strategies. First, we’re collaborating with a pharmaceutical company to evaluate the effects of ASK1 inhibitor treatments on MS and other diseases. Second, we’re pursuing gene therapy approaches. In MS, myelin in oligodendrocytes is destroyed by autoimmune reactions. Using gene therapy, we plan to increase amounts of activated TrkB in oligodendrocytes to see if this induces remyelination. Third, we’re examining cell replacement strategies using stem cells. I just came back from Kobe Eye Center, where I learned how to make retinal ganglion cells from stem cells. We plan on making retinal ganglion cells and oligodendrocytes from stem cells to determine whether addition of these cells may reduce disease symptoms. MS is an extremely painful disease for both patients and their families. My hope is to contribute to the development of new, good therapies to help these patients.
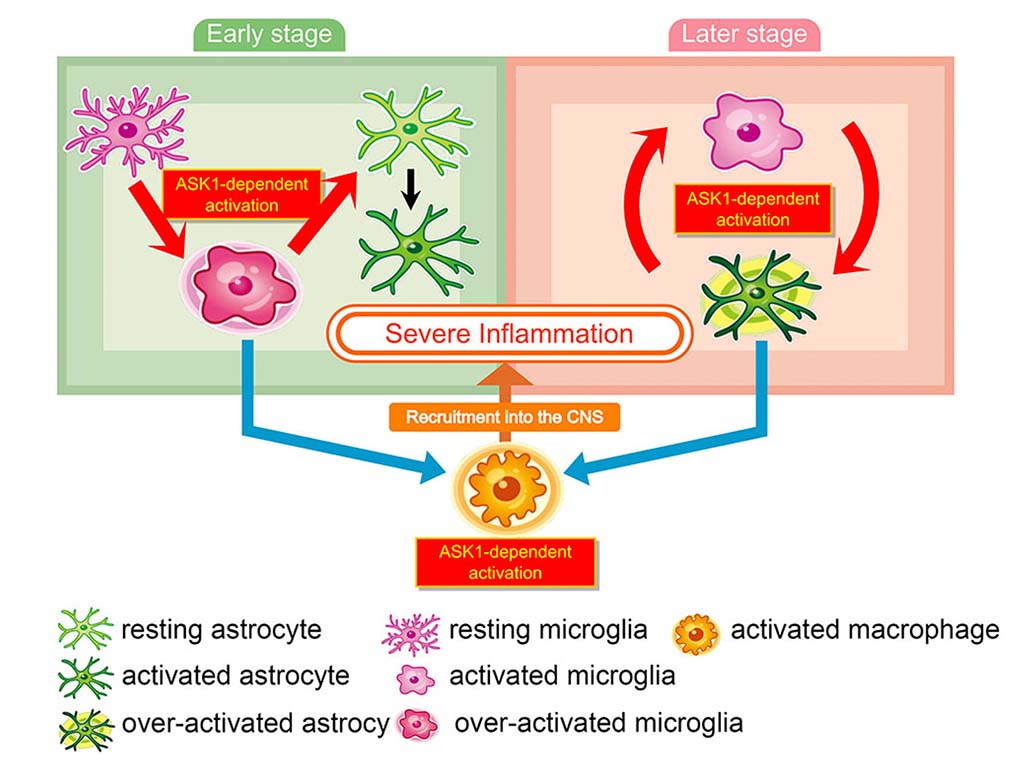
Schematic model of ASK1-mediated glial interaction during neuroinflammation.
Astrocytes and microglia activate each other in an ASK1-dependent manner. ASK1 signaling in microglia induces proinflammatory astrocytes in the early stage of EAE and ASK1 signaling in astrocytes induces proinflammatory microglia and recruit macrophages into the CNS in the late stage. ASK1-mediated activation of these glial cells causes sustained inflammation.
Interviewed by Jun Horiuch