Meet our scientists!
 BackTopics Page
BackTopics Page
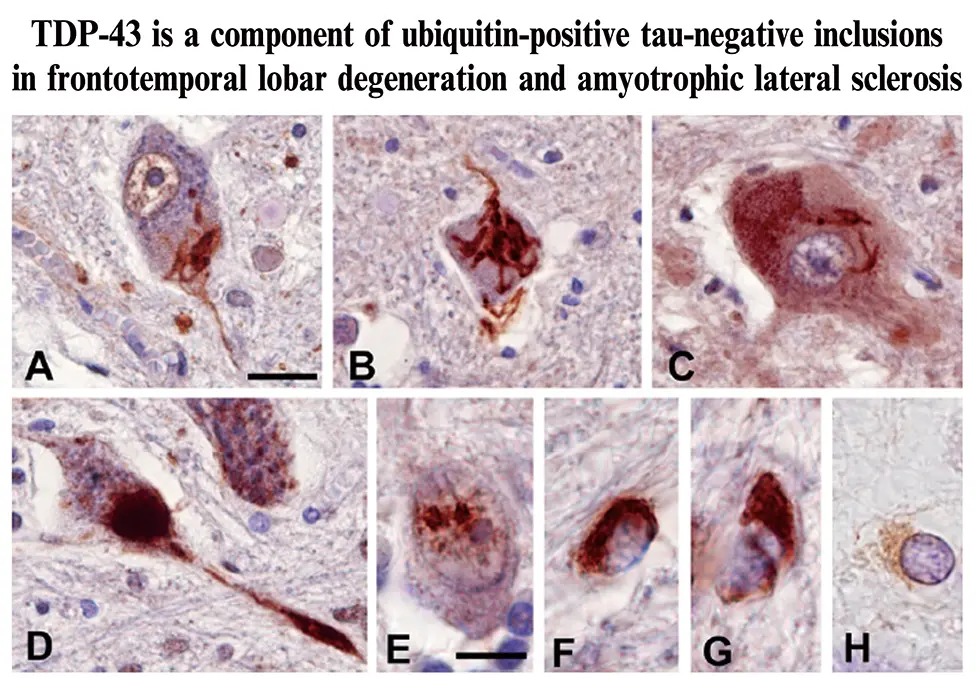
This year, we were thrilled that Dr. Masato Hasegawa, the head of the Dementia Research Project, was selected as a Clarivate laureate. Clarivate laureates are chosen among scientists who publish highly cited, high-impact papers that strongly influence research in their respective fields, and laureates are leading candidates for being awarded the Nobel Prize. Dr. Hasegawa was chosen based on his 2006 paper, “TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis,” as well as his continuing work on the propagation of neurodegerative diseases.

Neurodegenerative diseases can be separated into several different classes. For example, tauopathies including diseases such as Alzheimer’s disease, Pick’s disease, corticobasal degeneration, and progressive supranuclear palsy, are characterized by cytoplasmic inclusions containing insoluble tau aggregates, while synucleinopathies including diseases such as Parkinson’s disease and dementia with Lewy bodies, are characterized by inclusions containing α-synuclein aggregates. In his landmark paper, Dr. Hasegawa and colleagues determined that insoluble aggregates of a protein known as TDP-43, compose a third class of neurodegenerative disease which include frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS). Thus, three major pathogenic proteins, tau, α-synuclein, and TDP-43 are involved in major neurodegenerative diseases.
Dr. Hasegawa has studied these three proteins throughout his career in order to determine the difference between normal and abnormal versions, and elucidate how abnormal versions aggregate, accumulate in the brain, and spread to different regions of the brain. Early in his career, he characterized alterations in phosphorylation and ubiquitination in abnormal proteins and later helped propose the influential “prion-like propagation” hypothesis where abnormal proteins serve as seeds or templates that convert normal proteins to aberrant forms which then multiply and spread throughout the brain. To support this hypothesis, Dr. Hasegawa showed in multiple studies, both in vitro and in vivo, that normal proteins on their own very rarely convert to abnormal forms, while addition of a tiny amount of abnormal protein results in a much faster conversion to abnormal forms. The prion-like propagation hypothesis is currently the predominant theory for explaining disease progression. Dr. Hasegawa further showed that abnormal proteins spread through neuronal connections during disease progression.
What causes the differences between individual neurodegenerative diseases? There are at least ten distinct diseases associated with abnormal tau. Dr. Hasegawa and colleagues determined that different tauopathies were associated with different morphological tau filaments, and inoculating mice with different filaments induced pathologies characteristic of different diseases. He further collaborated in elucidating the crystal structures of tau filaments from different tauopathies and determined that different diseases had completely distinct filamentous structures.
Dr. Hasegawa’s work has contributed much to our understanding of the mechanisms that cause and propagate neurodegenerative diseases. While we do not yet have effective treatments to treat these diseases, elucidating mechanisms of disease progression is the first step in this process, and through his work and that of other scientists, we can hope for the development of effective treatments in the near future.
Written by Jun Horiuch