
- HOME
- Laboratory of Allergy and Immunology
Laboratory of Allergy and Immunology
Laboratory Head
Takachika Hiroi
Takachika Hiroi has been the leader of the laboratory of allergy and immunology since 2005. After graduation from Nihon university school of dentistry at Matsudo in 1986 (D.D.S.), he completed graduate school at Nihon university in 1990 (Ph.D). He started to work on mucosal immunology under the supervision of Dr. Hiroshi Kiyono at University of Alabama at Birmingham, Vaccine center, Alabama, USA, in 1992. After returning to Japan, he worked at Osaka University in 1995-2003 and at University Tokyo in 2003-2005. His current research is the study for effective bio-markers of sublingual immunotherapy (SLIT) for Japanese cedar pollen allergy . Further, the molecular mechanisms of mucosal tolerance still remain unclear. I want to elucidate this immune mechanism and develop drugs for some mucosal diseases in the future.
Research Summary
Recent Topics of Mucosal Immunology
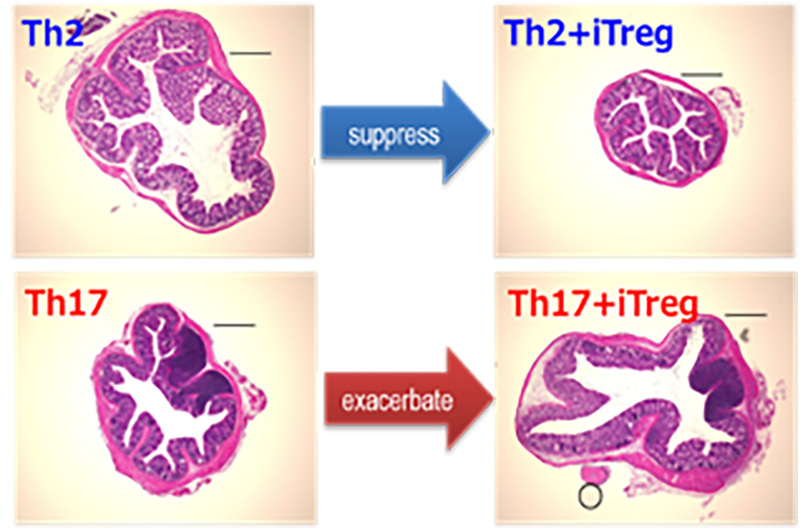
1. Antigen-specific iTreg cells stimulate Th17-mediated colon inflammation
CD4+ helper T cells play a crucial role in allergy and autoimmune diseases including inflammatory bowel diseases (IBDs). Th17 cells and Foxp3+ regulatory T cells (Tregs) are thought to promote and suppress inflammatory responses, respectively. Recently we have developed an antigen-specific and organ-targeted inflammation model by transferring antigen-specific helper T cell subsets followed by antigen administration. By adopting this strategy to colon, we have shown that antigen-specific Tregs stimulate Th17-mediated inflammation in a CTLA4-dependent manner. This finding will call for reconsideration of Treg/CTLA4-based immunological modulation to suppress or treat inflammatory diseases.
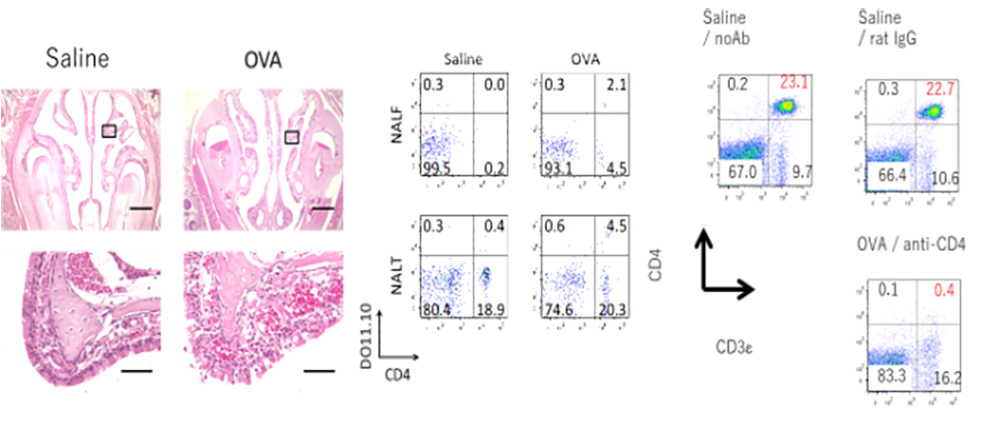
2. Essential Contribution of CD4+ T Cells to Antigen-Induced Nasal
Hyperresponsiveness in Experimental Allergic Rhinitis.
Recently, we have reported that CD4+ T cells play a crucial role in the pathogenesis of AR via induction of NHR, independent of IgE-, mast cell-, and eosinophil-mediated responses. (A) (B) Antigen-induced NHR in T cell-transferred mice. (C) Administration of an anti-CD4 mAb to immunized mice depleted peripheral CD4+ T cells almost completely.
Members
Laboratory Head Takachika Hiroi
- Mayumi Saeki
- Nobumasa Watanabe
- Tomoe Nshimura
Selected Publications
- Tachibana M, et al. (2020). “Ablation of IL-17A leads to severe colitis in IL-10-deficient mice: implications of myeloid-derived suppressor cells and NO production.” Int Immunol. 32: 187-201.
- Kitamura N, et al (2020). “Identification of novel interacting regions involving calcineurin and nuclear factor of activated T cells.” FASEB J. 34: 3197-3208.
- Takaiwa, F, et al. (2019). “Development of rice-seed-based oral allergy vaccines containing 2 hypoallergenic Japanese cedar pollen allergen derivatives for immunotherapy.” J Agric Food Chem. 67: 13127-13138.
- Kaminuma O, et al. (2018). “Downregulation of NFAT3 due to lack of T-box transcription factor TBX5 is crucial for cytokine expression in T cells.” J Immunol. 200: 92-100.
- Yokoyama S, et al. (2009) “Antibody-mediated blockade of IL-15 signaling reverses autoimmune intestinal damage in a mouse model of celiac disease.” Proc. Natl. Acad. Sci. USA 106: 15849-15854.