
HOME > Topics2019 > 6 November 2019
6 November 2019
Tetsuya Hirabayashi (Laboratory of Biomembrane) published a paper entitled "SDR9C7 catalyzes critical dehydrogenation of acylceramides for skin barrier formation" in The Journal of Clinical Investigation
SDR9C7 catalyzes critical dehydrogenation of acylceramides for skin barrier formation
- <Title of the paper>
- SDR9C7 catalyzes critical dehydrogenation of acylceramides for skin barrier formation
- <Journal>
- The Journal of Clinical Investigation
DOI: 10.1172/JCI130675
https://www.jci.org/articles/view/130675
Summary
Prof. Masashi Akiyama, Dr. Takuya Takeichi at Department of Dermatology, Nagoya University Graduate School of Medicine*, Prof. Alan R. Brash at Department of Pharmacology and the Vanderbilt Institute of Chemical Biology, Vanderbilt University, and Dr. Tetsuya Hirabayashi at Laboratory of Biomembrane, Tokyo Metropolitan Institute of Medical Science, discovered that SDR9C7 catalyzes critical dehydrogenation of acylceramides for skin barrier formation.
SDR9C7 encodes a short chain dehydrogenase/reductase family 9C member 7 (SDR9C7), recently found mutated in ichthyosis. In a patient with SDR9C7 mutation and a mouse Sdr9c7 knockout model, we showed loss of covalent binding of epidermal ceramides to protein, a structural fault in the corneocyte lipid envelope essential to the skin barrier. For reasons unresolved, protein binding requires lipoxygenase-catalyzed transformations of linoleic acid (18:2) esterified in ω-O-acylceramides. In Sdr9c7–/– epidermis, quantitative LC-MS assays revealed almost complete loss of a species of ω-O-acylceramides esterified with linoleate-9,10-trans-epoxy-11E-13-ketone; other acylceramides related to the lipoxygenase pathway were in higher abundance. Recombinant SDR9C7 catalyzed NAD+-dependent dehydrogenation of linoleate 9,10-trans-epoxy-11E-13-alcohol to the corresponding 13-ketone, while ichthyosis mutants were inactive. We propose, therefore, that the critical requirement for lipoxygenases and SDR9C7 is in producing acylceramide containing the 9,10-epoxy-11E-13-ketone, a reactive moiety known for its non-enzymatic coupling to protein. This suggests a novel mechanism for coupling of ceramide to protein and provides important insights into skin barrier formation and ichthyosis pathogenesis.
Research Background
The corneocyte lipid envelope composed of covalently bound ceramides and fatty acids is important to the integrity of the permeability barrier in the stratum corneum, and its absence is a prime structural defect in various skin diseases associated with defective skin barrier function. In 2016, two missense mutations in SDR9C7 were identified in patients with autosomal recessive congenital ichthyosis (ARCI), and several more in 2018. SDR9C7 encodes a short chain dehydrogenase/reductase family 9C member 7 (SDR9C7), and is highly expressed in the epidermis. Our ultrastructural observations of the skin lesion of an ARCI patient with SDR9C7 mutations revealed disruption of the intercellular lipid layers in the stratum corneum.
Research Results
In a patient with SDR9C7 mutation and a mouse Sdr9c7 knockout model, we showed loss of covalent binding of epidermal ceramides to protein, a structural fault in the corneocyte lipid envelope essential to the skin barrier. For reasons unresolved, protein binding requires lipoxygenase-catalyzed transformations of linoleic acid (18:2) esterified in ω-O-acylceramides. In Sdr9c7–/– epidermis, quantitative LC-MS assays revealed almost complete loss of a species of ω-O-acylceramides esterified with linoleate-9,10-trans-epoxy-11E-13-ketone; other acylceramides related to the lipoxygenase pathway were in higher abundance. Recombinant SDR9C7 catalyzed NAD+-dependent dehydrogenation of linoleate 9,10-trans-epoxy-11E-13-alcohol to the corresponding 13-ketone, while ichthyosis mutants were inactive.
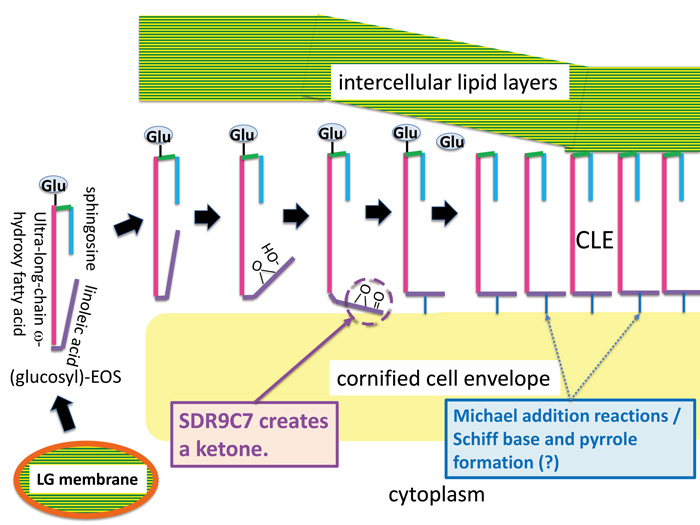
Research Summary and Future Perspective
We propose that the critical requirement for lipoxygenases and SDR9C7 is in producing acylceramide containing the 9,10-trans-epoxy-11E-13-ketone, a reactive moiety known for its non-enzymatic coupling to protein. This suggests a mechanism for coupling of ceramide to protein and provides important insights into skin barrier formation and ichthyosis pathogenesis.
Publication
Takuya Takeichi, Tetsuya Hirabayashi, Yuki Miyasaka, Akane Kawamoto, Yusuke Okuno, Shijima Taguchi, Kana Tanahashi, Chiaki Murase, Hiroyuki Takama, Kosei Tanaka, William E. Boeglin, M. Wade Calcutt, Daisuke Watanabe, Michihiro Kono, Yoshinao Muro, Junko Ishikawa, Tamio Ohno, Alan R. Brash, Masashi Akiyama. SDR9C7 catalyzes critical dehydrogenation of acylceramides for skin barrier formation. The Journal of Clinical Investigation, published online before print October 31, 2019.


