Stem cell project team in our institute revealed that in vitro production of hematopoietic progenitor cells from human induced pluripotent stem cells was enhanced by interferon-γ/FLT3 pathway. This study was published as an original research article in US academic journal.

Human induced pluripotent stem cells (hiPSCs; Figure 1) could be differentiated into various cell types when cultured in specialized conditions. These differentiated cells are useful for regenerative medicine, drug discovery, and pathological studies. It has been known that hematopoietic stem/progenitor cells (HSPCs) could be obtained from hiPSCs by several culture methods. An important feature of HSPCs is bone marrow repopulating activity [1], however, the repopulating activity of hiPSC-derived HSPCs were significantly low. To obtain highly-potent HSPCs from hiPSCs, great efforts have been made in the world.
HSPCs are originated from aorta/gonad/mesonephros (AGM) region during mammalian embryogenesis. By mimicking the embryonic HSPC developmental process by hiPSC-derived organoids [2] (Figure 1), we could obtain hematopoietic cells expressing HSPC markers from hiPSCs. However these HSPC-like cells showed low repopulating activity when transplanted into conditioned immune-compromised mice.
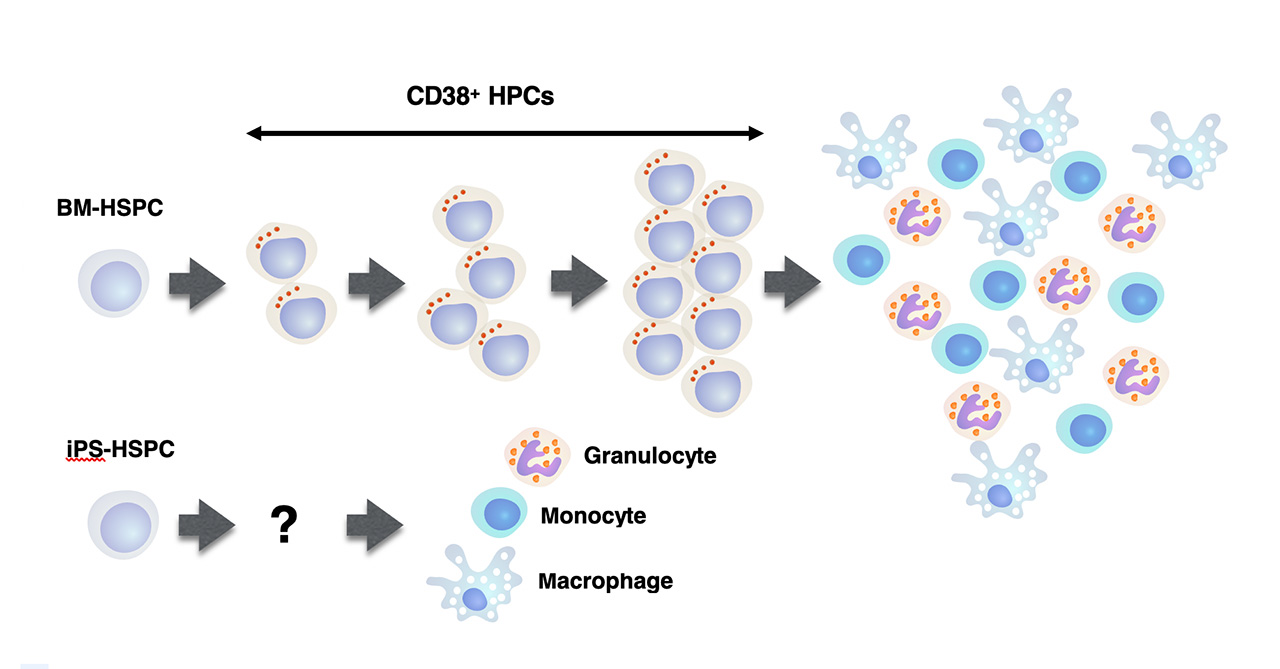
Subsequently these HSPCs were thoroughly investigated to seek their fundamental defects. In bone marrow, HSPCs are differentiated into mature myeloid cells via hematopoietic progenitor cells (HPCs) expressing CD38 on their cell surfaces (Figure 2). These CD38+ HPSCs exhibit highly proliferative potential, hence the small number of HSPCs could give rise to huge number of myeloid cells via multiple cell division steps at the CD38+ HPC stage (Figure 2). Notably, we found that hiPSC-derived HPSCs were differentiated into myeloid cells without or rapidly passing through CD38+ HPC stage (Figure 2). Possibly, this unusual defect would be linked with their low engraftable activity.
We next found that the expression of FLT3 was significantly low in the iPS-derived HSPCs when compared with cord blood HSPCs. FLT3 encodes a receptor tyrosine kinase expressed on the cell surface. FLT3 is activated by FLT3L, a cytokine supporting cell proliferation of HSPCs. Thus FLT3 is required for the transduction of mitogenic stimulation from FLT3L. Therefore we examined the effects of enforced FLT3 expression on hematopoietic induction from hiPSCs in the presence of FLT3L. Consequently, FLT3 expression unaffected the number of HSPCs while CD38+ HPCs were significantly increased. Therefore the absence of CD38+ HPCs was caused by the failed expression of FLT3.
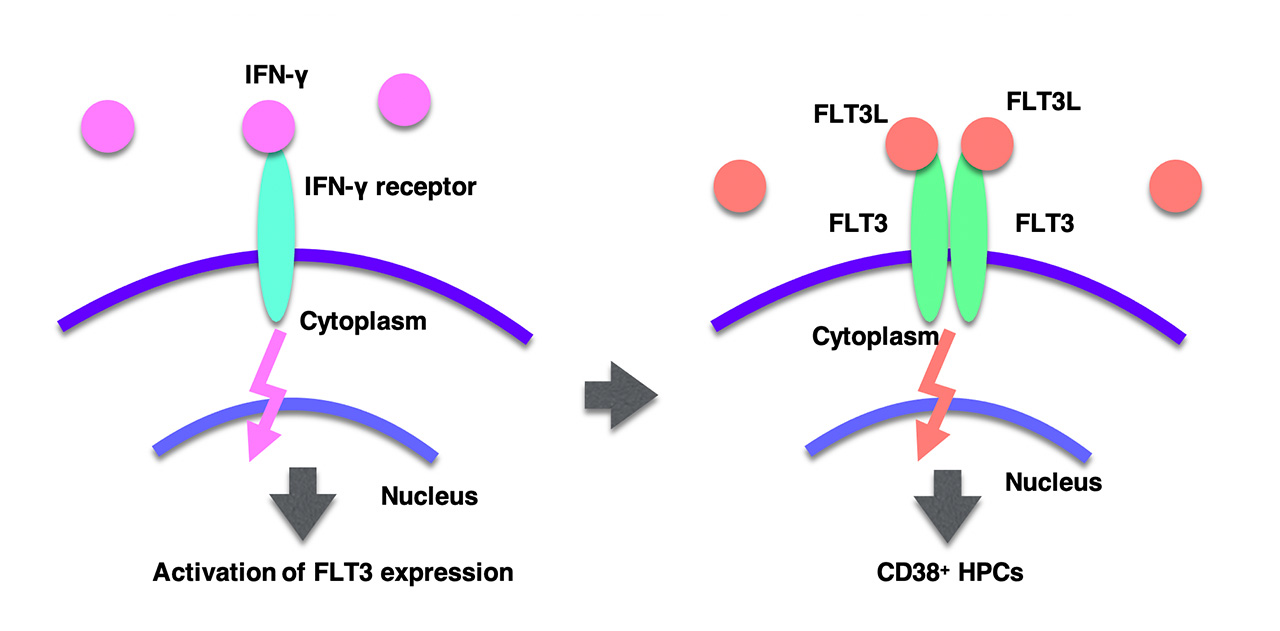
By searching several cytokines/chemicals, we found that interferon-γ (IFN-γ) could induce FLT3 expression in hiPSC-derived HSPCs (Figure 3). Furthermore IFN-γ treatment drastically increased CD38+ HPCs from hiPSCs (Figure 3). Therefore it would be plausible that inflammatory signals, such as IFN-γ, is required for establishment of adult-type hematopoietic hierarchy including CD38+ HPCs. However, FLT3 and IFN-γ did not enhance bone marrow populating ativity of hiPSC-derived HSPCs. Thereby other cue(s) would be required to obtain highly-potent HSPCs from hiPSCs. On the other hand, we found that a chemical compound UM171, known to support ex vivo amplification of cord blood HSPCs, was effective for in vitro expansion of hiPSC-derived HSPCs. Thus, UM171-mediated in vitro expansion of hiPSC-derived HSPCs would be useful as a stem cell source for various hematopoietic cells.