
- HOME
- Visual Research Project
Visual Research Project
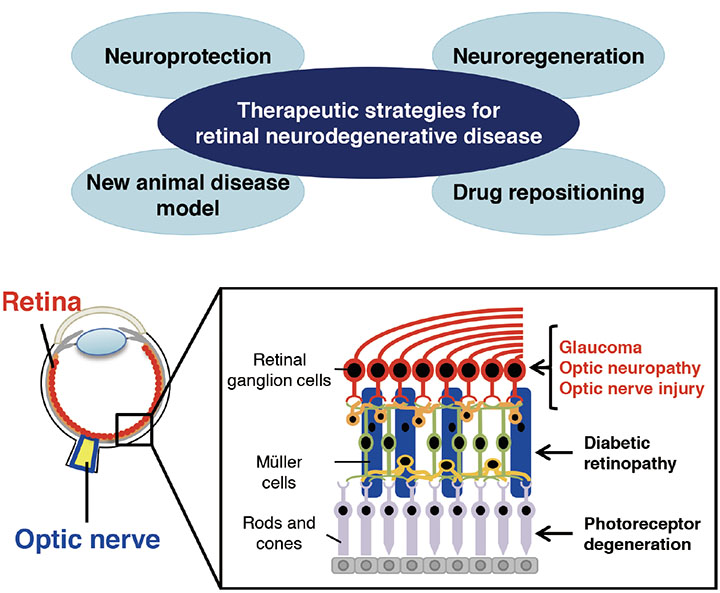
Development of Therapeutic Strategies for Visual Impairment using Neuroprotection and Optic Nerve Regeneration
Research Summary
More than 1.6 million people in Japan are visually impaired and the number of patients with conditions such as glaucoma and diabetic retinopathy is increasing. We seek to elucidate mechanisms involved in the onset of visual impairments such as optic neuritis, develop a neuroprotective retinal therapy using animal disease models, and establish methods to promote regeneration of the optic nerve.
Apoptosis signal related kinase 1 (ASK1) is a mitogen-activated protein kinase kinase kinase that has been shown to cause neuroinflammation, but its mechanism of action has been unclear. We generated conditional knockout mice that lack ASK1 in immune cells or glial cells to assess the cell-type-specific roles of ASK1 in experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS). We found that ASK1 is required in microglia and astrocytes to cause and maintain neuroinflammation by a proinflammatory feedback loop between these two cell types. Disruption of this feedback loop by suppression of glial ASK1 may be a novel and effective approach for reducing neuroinflammation.
We have also been examining the role of DOCK-D family proteins in neuroinflammation.
DOCK proteins are atypical guanine nucleotide exchange factors, and we found that deficiencies in DOCK10 reduced neuroinflammation in EAE. Thus, inhibition of DOCK10 may be useful for treatment of diseases such as MS and optic neuritis.
The Rho-ROCK pathway regulates actin cytoskeleton and dynamics, and we have recently reported that application of the Rho-ROCK inhibitor ripasudil eyedrops promoted optic nerve regeneration and neuroprotection by suppressing phosphorylation of CRMP2 and cofilin, two proteins involved in the Rho-ROCK pathway.
Selected Publications
- Nishijima E, Honda S, Kitamura Y, Namekata K, Kimura A, Guo X, Azuchi Y, Harada C, Murakami A, Matsuda A, Nakano T, Parada LF, Harada T (2023) “Vision protection and robust axon regeneration in glaucoma models by membrane-associated Trk receptors." Molecular Therapy 31(3), 810-824.
- Inoue-Yanagimachi M, Himori N, Uchida K, Tawarayama H, Sato K, Yamamoto M, Namekata K, Harada T, Nakazawa T (2023) “Changes in glial cells and neurotrophic factors due to rotenone-induced oxidative stress in Nrf2 knockout mice.” Experimental Eye Research 226, 109314.
- Shinozaki Y, Leung A, Namekata K, Saitoh S, Nguyen HB, Takeda A, Danjo Y, Morizawa Y, Shigetomi E, Sano F, Yoshioka N, Takebayashi H, Ohno N, Segawa T, Miyake K, Kashiwagi K, Harada T, Ohnuma S, Koizumi S (2022) "Astrocytic dysfunction induced by ABCA1 deficiency causes optic neuropathy.” Science Advances 8(44), eabq1081.
- Guo X, Kimura A, Namekata K, Harada C, Arai N, Takeda K, Ichijo H, Harada T (2022) “ASK1 signaling regulates phase-specific glial interactions during neuroinflammation.” PNAS 119(6), e2103812119.
- Brahma MM, Takahashi K, Namekata K, Harada T, Goshima Y, Ohshima T (2022) “Genetic inhibition of collapsin response mediator protein-2 phosphorylation ameliorates retinal ganglion cell death in normal-tension glaucoma models.” Genes to Cells 27(8), 526-536.
- Ohashi T, Namekata K, Guo X, Kimura A, Harada C, Harada T (2022) “Effects of lighting environment on the degeneration of retinal ganglion cells in glutamate/aspartate transporter deficient mice, a mouse model of normal tension glaucoma.” Biochemistry and Biophysics Reports 29, 101197
- Sano H, Namekata K, Niki M, Semba K, Murao F, Harada T, Mitamura Y (2022) “Ocular expression of cyclin-dependent kinase 5 in patients with proliferative diabetic retinopathy.” Journal of Diabetes Investigation 13(4), 628-637
