2025
- Nov. 10-11, 2025: Dr. Maruyama gave a lecture as an invited speaker, trainee Sugita and Yamazaki gave a poster presentation at AMED International Symposium 2025 ~ The Early-Life Nexus, Keio University - Mita Campus.
- Oct. 22, 2025: Trainee Sugita and Yamazaki gave a presentation, and Sugita received the Best Presentation Award at the institute's research presentation meeting.(Photo)
- Sept. 11 -13, 2025: Trainee Wada and Sugita gave a presentation, Sugita received the Excellent Presentation Award at the 68th Annual Meeting of the Japanese Society for Neurochemistry.(Photo)
- July 30-Augst 1, 2025: We held a summer seminar on brain development and evolution for high school students. (Photo)
- July 24-27, 2025: Dr. Maruyama attended, researcher Hatanaka and trainees Yamazaki and Shinohara gave a poster presentation at the 48th Annual Meeting of the Japan Neuroscience Society. (Photo)
- July 14-16, 2025: Dr. Maruyama gave an oral presentation as an invited speaker and trainee Achiwa gave a poster presentation at the Anatomical Society Summer Meeting held at St. John's College, University of Oxford, UK. (Photo)
- June 27-28, 2025: The 4th research group meeting of AMED-CREST “Study on the dynamism of subplate neural activity during brain development” was held at TMIMS. (Photo)
- June 4-8, 2025: Dr. Maruyama gave an oral presentation as an invited speaker at Black Sea Neurogenesis 2025, Bulgaria. (Photo)
- June 2-3, 2025: Dr. Maruyama gave a lecture as a Neuroscience Theme Guest Speaker at Sherrington Library, University of Oxford, UK. (Photo)
- May 25-28, 2025: Dr. Maruyama gave an oral presentation, researchers Moriya and Matsumura, and trainee Sugita gave a poster presentation at Cortical Development Conference 2025, Sicily, Italy. Trainee Sugita received the Best Question Award. (Photo)
- April 15-16, 2025: Dr. Maruyama, Dr. Kumamoto and Prof. Molnar organized the 30thTMIM International Symposium “Principles of Neocortical Development and Evolution II” at our institute. (Photo)
- April, 2025: Dr. Maruyama, Dr. Kumamoto and former researcher Hara at Research Center for Genome & Medical Science published a paper in Scientific Reports on “The spatial transcriptome of the late-stage embryonic and postnatal mouse brain reveals spatiotemporal molecular markers.”
- April, 2025: Dr. Maruyama and guest researcher Nomura published a paper in Development, Growth & Differentiation on “Genetic and developmental bases for mammalian neocortical evolution.”
- April 3-5, 2025: Dr. Maruyama and Prof. Molnar gave a lecture at Brain Development Symposium Kyoto 2025, hosted by Kyoto Women’s University. (Photo)
- March, 2025: Prof. Zoltan Molnar gave a lecture at TMIMS Seminar as follows: (Photo)
- March 10: Development and Evolution of Thalamocortical Connectivity
- March 27: Transient Circuits in the Developing Brain
- March, 2025: Prof. Zoltan Molnar gave anatomy lecture series at TMIMS as follows: (Photo)
- March 13: Lectures on neuroanatomy-I: Thomas Willis (1621-1675) The Founder of Neuroanatomy and Clinical Neurology
- March 26: Lectures on neuroanatomy-II: Insight into the Life and Work of Sir Charles Sherrington(1857-1952)
- April 9: Lectures on neuroanatomy-III: Evolutionary Developmental Biology of the Mammalian Cerebral Cortex
- March 9-May 7, 2025: Dr. Maruyama invited Prof. Zoltan Molnar from the University of Oxford, as a part of Foreign Researcher Invitation Program. (Photo)
- March 12, 2025: Trainee Nomura received the Excellent Presenter Award at the 3rd section of the institute's research presentation meeting. (Photo)
- Feb. 22, 2025: Dr. Maruyama gave a lecture titled “What is the brain and how is it made?” to the general public at the 8th Tomin Koza (public lecture series) on “How the brain was born and evolved - the evolutionary path to the human brain and disease.” (Photo)
- Jan. 16-17, 2025: Dr. Maruyama gave an oral presentation online, researcher Matsumura gave a poster presentation at AMED Area Conference, Fukuoka.
2024
- Nov. 27-29, 2024: Dr. Maruyama gave an oral presentation as an invited speaker and trainees Song, Nomura, Ishizuka and Doi gave a poster presentation at the 47th Annual Meeting of the Molecular Biology Society of Japan.
- Sept. 12, 2024: Dr. Maruyama gave an oral presentation as an invited speaker at Evolution and Development of Nervous System, Croatia.
- July 31- Augst 2: We held a summer seminar on brain development and evolution for high school students. (Photo)
- July 24-27, 2024: Dr. Maruyama and trainee Sugita gave an oral presentation and researcher Moriya and trainees Achiwa and Wada gave a poster presentation at the 47th Annual Meeting of the Japan Neuroscience Society.
- June 23, 2024: Prof. Debra Lynn Silver of Duke University held a seminar at the TMIMS. She gave technical guidance to the researchers. (Photo)
- June 14-15, 2024: The 3rd research group meeting of AMED-CREST “Study on the dynamism of subplate neural activity during brain development” was held at TMIMS.(Photo)
- June 13, 2024: Dr. Maruyama and former trainee Kaneko published a paper in EMBO reports on “ADAMTS2 promotes radial migration by activating TGF-βsignaling in the developing neocortex.”
- May 9, 2024: Dr. Maruyama, trainee Katayama and colleagues published a paper in the Journal of Comparative Neurology on “Thalamic activity-dependent specification of sensory input neurons in the developing chick entopallium.”
- April 19, 2024: Dr. Maruyama received the Prize for Science and Technology (Research Category) by the Minister of Education, Culture, Sports, Science and Technology for 2024. (Photo)
- March 16, 2024: Trainee Katayama received the Best Presentation Award at the 76th Annual Meeting of the Kanto Branch of the Zoological Society of Japan.(Photo)
- March 11, 2024: Trainee Morioka received the Excellent Presenter Award at the 3rd section of the institute's research presentation meeting.
- February 17, 2024: Trainee Sugita received the Best Presentation Award at the winter seminar co-hosted by the Kinki and Chugoku-Shikoku Chapters of the Young Researchers Society of Biochemistry.
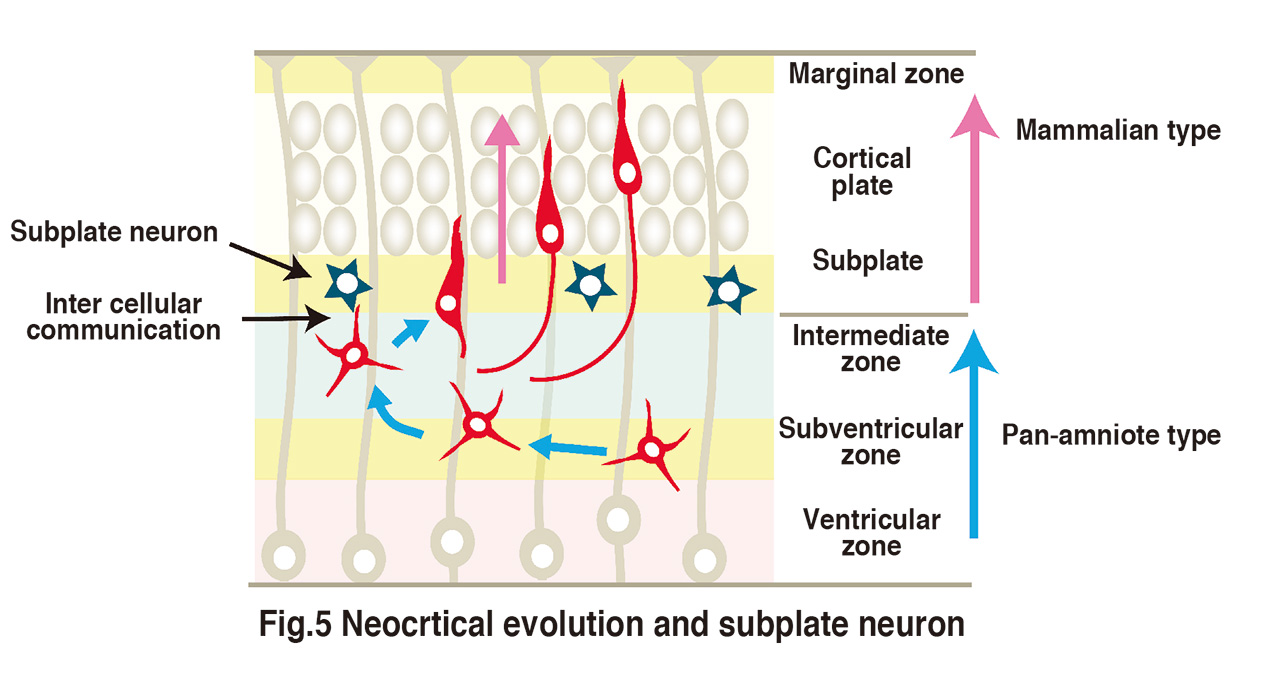
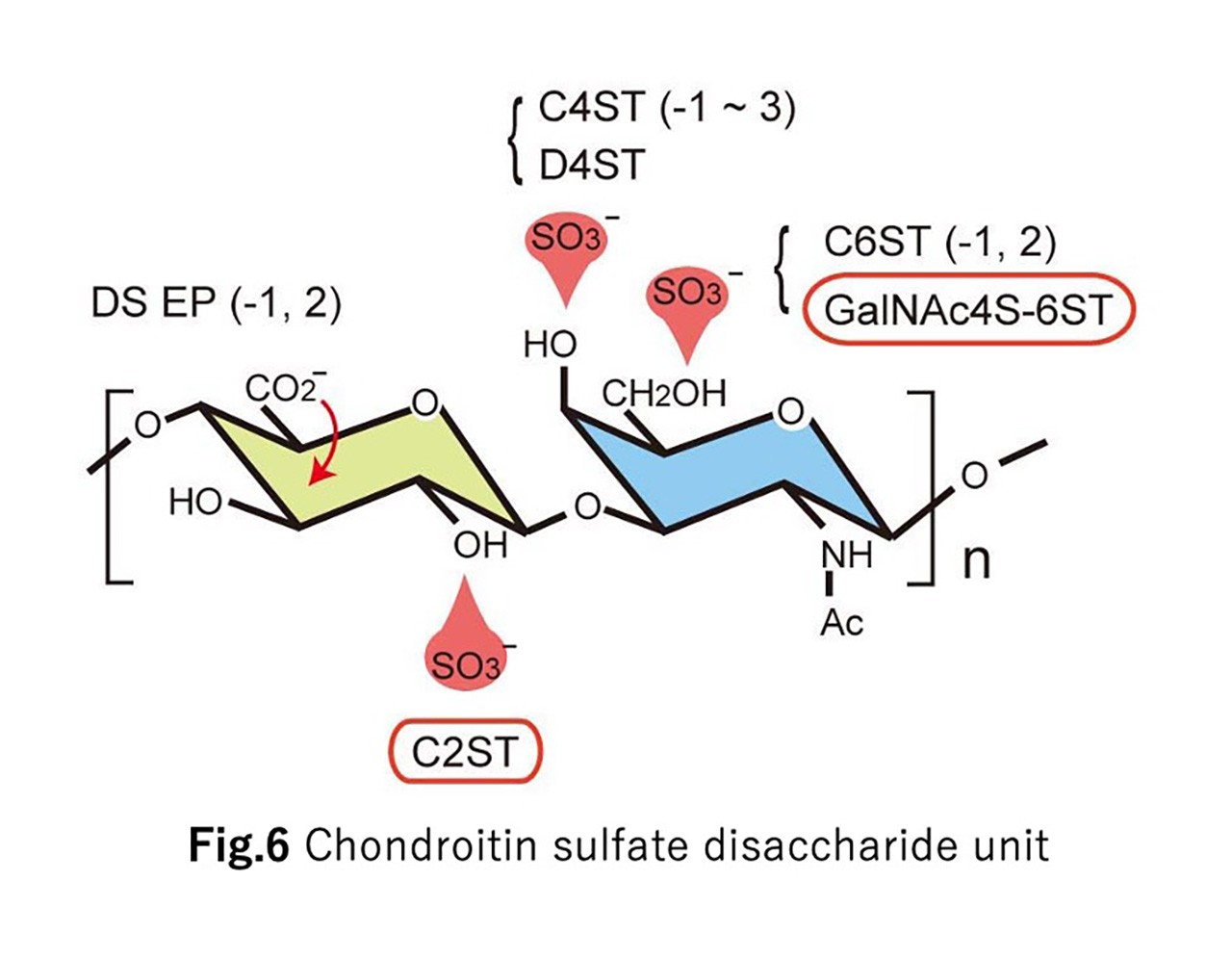
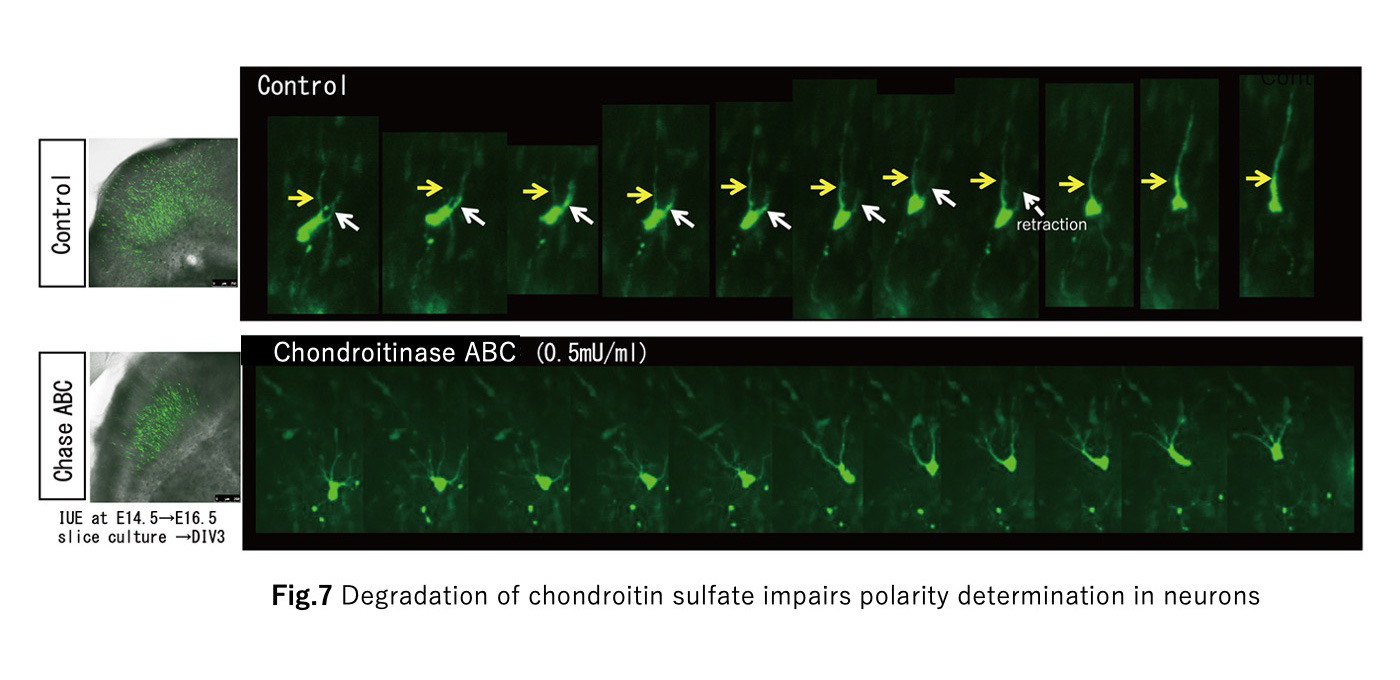
How are the elaborate neural circuits formed during the development?
This is a profound problem that continues to present new challenges. Neural circuit formation proceeds under diverse cell-cell and cell-extracellular matrix interactions. This project aims to elucidate the molecular mechanisms of such interactions using the mouse cerebral cortex and the Drosophila neuromuscular junction as models.