
Toriumi K, Miyashita M, Suzuki K, Yamasaki N, Yasumura M, Horiuchi Y, Yoshikawa A, Asakura M, Usui N, Itokawa M, Arai M. Transl Psychiatry. 2021 May 3;11(1):262. doi: 10.1038/s41398-021-01381-z.PMID: 33941768
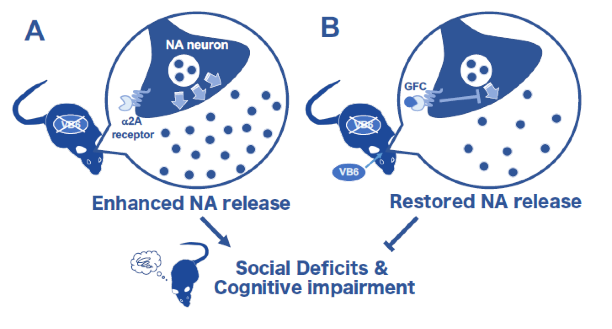
(A) VB6(-) mice showed hyperactivate noradrenergic (NAergic) signaling, resulting in behavioral deficits comparable to schizophrenia. (B) These are ameliorated by VB6 supplementation into the brain or treatment with the α2A adrenoreceptor agonist guanfacine (GFC), which suppresses NA release.
Schizophrenia is a heterogeneous psychiatric disorder characterized by positive symptoms such as hallucinations and delusions, negative symptoms such as apathy and lack of emotion, and cognitive impairment. We have reported that VB6 (pyridoxal) levels in peripheral blood of a subpopulation of patients with schizophrenia is significantly lower than that of healthy controls. More than 35% of patients with schizophrenia have low levels of VB6 (clinically defined as male: < 6 ng/ml, female: < 4 ng/ml). VB6 level is inversely proportional to severity score on the positive and negative symptom scale (PANSS), suggesting that VB6 deficiency might contribute to the development of schizophrenia symptoms. In fact, a recent review has shown the decreased VB6 in patients with schizophrenia as the most convincing evidence in peripheral biomarkers for major mental disorders. Additionally, we recently reported that high-dose VB6 (pyridoxamine) was effective in alleviating psychotic symptoms, particularly the PANSS negative and general subscales, in a subset of patients with schizophrenia. Although a link between lower VB6 level and schizophrenia is widely hypothesized, the mechanism behind this remains poorly understood.
VB6 is not synthesized de novo in humans, but is primarily obtained from foods. In the present study, to clarify the relationship between VB6 deficiency and schizophrenia, we generated VB6-deficient (VB6(-)) mice through feeding with a VB6-lacking diet as a mouse model for the subpopulation of schizophrenia patients with VB6 deficiency.
After feeding for 4 weeks, plasma VB6 level in VB6(-) mice decreased to 3% of that in control mice. The VB6(-) mice showed social deficits and cognitive impairment. Furthermore, the VB6(-) mice showed a marked increase in 3-methoxy-4-hydroxyphenylglycol (MHPG) in the brain, suggesting enhanced NA metabolism in VB6(-) mice (Fig. 2A-C). We confirmed the increased NA release in the prefrontal cortex and the striatum of VB6(-) mice through in vivo microdialysis (Fig. 2D). These findings suggest that the activities of NAergic neuronal systems are enhanced in VB6(-) mice.
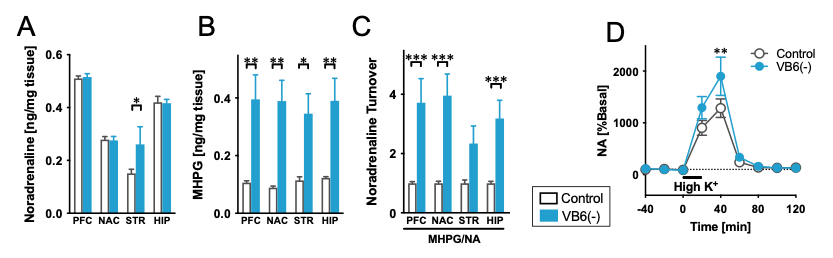
(A) NA, (B) MHPG, and (C) NA turnover in each brain region of VB6(-) mice were measured. (D) NA release in the prefrontal cortex was measured. High K+ stimulation was performed at the time point of 0 min. VB6(-) mice showed hyperactivate NAergic signaling.
Furthermore, VB6 supplementation directly into the brain using an osmotic pump ameliorated the hyperactivation of the NAergic system and behavioral abnormalities (Fig.3A-C). indicating that the enhanced NA turnover and the behavioral deficits shown in the VB6(-) mice are attributed to VB6 deficiency in the central nervous system. In addition, the α2A adrenergic receptor agonist guanfacine also improved the hyperactivated NAergic system in the frontal cortex and behavioral disorders (Fig. 3D-F). These results show that the behavioral deficits in VB6(-) mice may be caused by an enhancement of NAergic signaling.
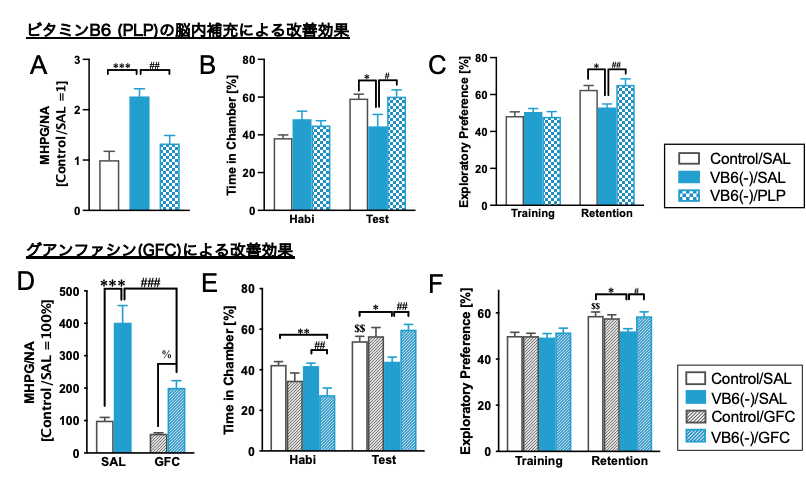
(A)(D) The NA turnover in the PFC were assessed. The results in (B)(E) social interaction test and (C)(F) novel object recognition test were shown. The VB6 supplementation into the brain and guanfacine ameliorated the enhanced NAergic system and the behavioral deficits observed in VB6(-) mice.
Schizophrenic patients with VB6 deficiency, who account for more than 35% of all patients, present with relatively severe clinical symptoms and treatment resistance. Our findings suggest that a new therapeutic strategy targeting the NAergic system might be effective for these patients. They will also provide evidence based on pathophysiology for a new therapeutic strategy called "VB6 treatment for schizophrenia," which we are currently conducting clinical research on.