
Iino K, Toriumi K, Agarie R, Miyashita M, Suzuki K, Horiuchi Y, Niizato K, Oshima K, Imai A, Nagase Y, Kushima I, Koike S, Ikegame T, Jinde S, Nagata E, Washizuka S, Miyata T, Takizawa S, Hashimoto R, Kasai K, Ozaki N, Itokawa M, Arai M. Front Genet. 2021 Dec 6;12:762999.
doi: 10.3389/fgene.2021.762999.
The accumulation of GlucA might be related to drug-resistant schizophrenia, since GlucA is known to promote drug excretion by forming conjugates with drugs. However, little is known about the molecular mechanisms underlying GlucA accumulation in patients with schizophrenia.
AKR1A1 is an approximately 40 kDa monomeric oxidoreductase, which is known to catalyze the reduction of GlucA. It can be assumed that AKR1A1 dysfunction leads accumulation of GlucA. Here, we aimed to explore genetic defects in AKR1A1 in patients with schizophrenia and identify the molecular mechanisms that cause the accumulation of GlucA.
The AKR1A1 sequence was analyzed in patients with schizophrenia and control subjects, and 28 variants containing 4 novel variants were identified (Fig.1). Among them, four variants were found in the coding region. In particular, the c.753G>A variant was identified in 14 cases in the schizophrenia patient group and 5 subjects in the healthy group, and the c.264delC variant was observed in only one patient with schizophrenia.
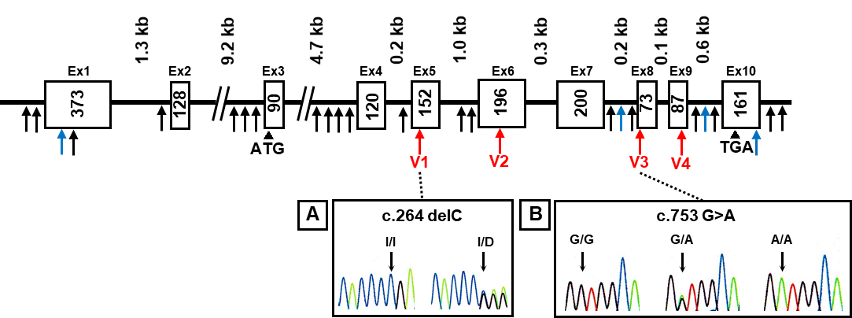
Novel variants (blue arrows), variants in coding regions (V1–V4 variants: red arrows), and others (black arrows) are shown. Heterozygous sequence traces derived from individuals carrying (A) a cytosine deletion within exon 5 (V1 variant) and (B) a mutation from guanine to adenine at the first position of exon 8 (V3 variant).
A mutation at the first position of an exon has been reported to cause exon skipping by alternative splicing. To address whether the variant c.753G>A (V3 variant) located at the first position of exon 8 in the AKR1A1 gene results in exon skipping, a minigene assay was performed. The results showed that exon 8 skipping occurred in the A allele minigene (mutant [MT]) but not in the G allele (WT) in any cell type (Fig. 2A-C). In HEK293 and SH-SY5Y cells, MT considerably increased the frequency of exon skipping compared to WT (Fig.2C). The skipping of exon 8 causes a frameshift mutation and may result in the decreased enzymatic activity of AKR1A1 due to the c.753G>A variant, even though it is a silent mutation. To confirm this, we purified the AKR1A1 mutant recombinant protein produced by exon skipping and evaluated its activity. We found that AKR1A1 produced by the c.753G>A variant and c.264delC exhibited no activity, which was significantly lower than that of the WT AKR1A1 (Fig.2D, E).
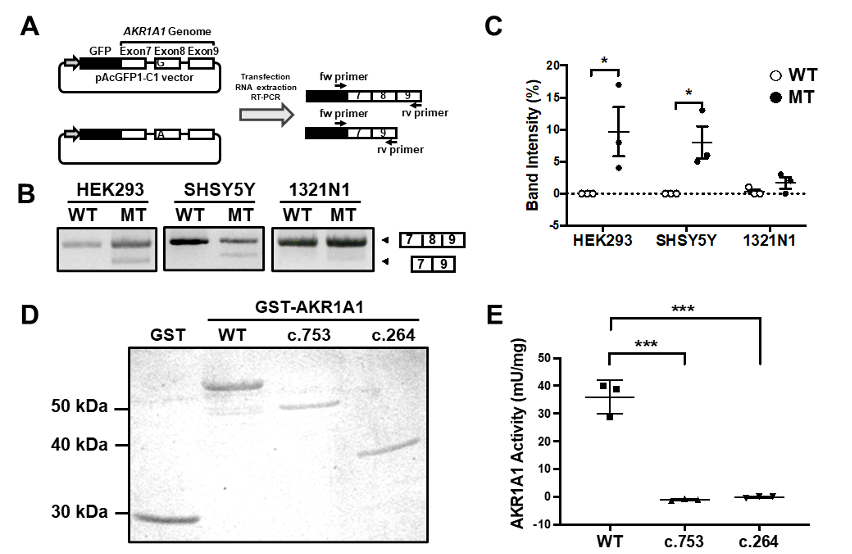
A) An outline of the splicing assay is shown. Exon 8 skipping was confirmed using cDNA generated from HEK293, SH-SY5Y, and 1321N1 cells expressing minigenes for WT or c.753G>A variant (mutant [MT]). (B) The results of the splicing assay are shown. The upper band indicates products including exons 7–9, whereas the lower band shows exon 8 skipping products, including only exons 7 and 9. (C) Data represent the mean of three independent experiments for the splicing assay. (D) GST and GST-AKR1A1s were purified and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. (E) AKR1A1 activity of the purified GST-AKR1A1s was determined. AKR1A1 produced by the c.753G>A variant and c.264delC exhibited decreased enzymatic activity.
To investigate the effect of the c.753G>A variant on AKR1A1 enzymatic activity in patients with schizophrenia, AKR enzymatic activity in red blood cells from six patients with schizophrenia (SCZ#1~SCZ#6) and two control subjects (CON#1, CON#2) was measured. The enzymatic activity in four patients with GA (SCZ#1~SCZ#4) was slightly lower than that in four subjects with GG (SCZ#5, SCZ#6, CON#1, CON#2) (Fig.3A). Finally, we quantified mRNA level in the whole blood cells obtained from five subjects (CON#2 and SCZ#6 with c.753 GG alleles, and SCZ#1, SCZ#2, and SCZ#4 with c.753 GA alleles). We found that mRNA expression of AKR1A1 with c.753 GA alleles decreased to approximately 50% compared to that with c.753 GG (Fig.3B). These results suggest that the reduction of AKR activity in subjects with the variant may be caused from the reduction of gene expression.
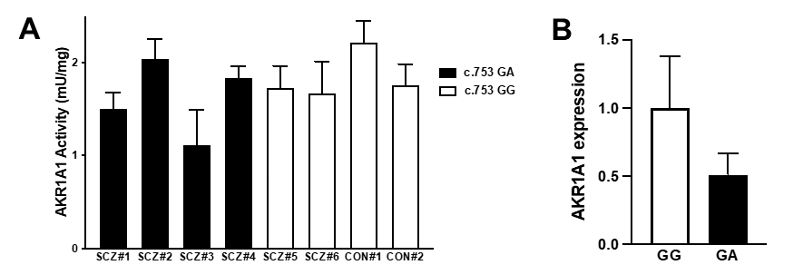
(A) The enzymatic activities of AKR in the red blood cells of six patients with schizophrenia (SCZ#1 to #4 with the c.753G>A variant and SCZ#5 and #6 without the variant) and two control subjects (CON#1 and #2 without the variant) were examined. The enzymatic activity in patients with GA was slightly lower than that in subjects with GG. (B) The AKR1A1 mRNA expression in five subjects (SCZ#1, SCZ#3, SCZ#4, SCZ#6, and CON#2) was quantified by qPCR. The mRNA expression of AKR1A1 with c.753 GA alleles was decreased to approximately 50% compared to that with c.753 GG.