
Ishida.H, Miyashita M., Oshima K., Kawakami I., Sekiyama K., Kounoe M., Seki E., Arai N., Takizawa S., Nagata E., Itokawa M., Arai M. Psychiatry Res Case Rep. (in Press). doi: 10.1016/j.psycr.2022.100064.
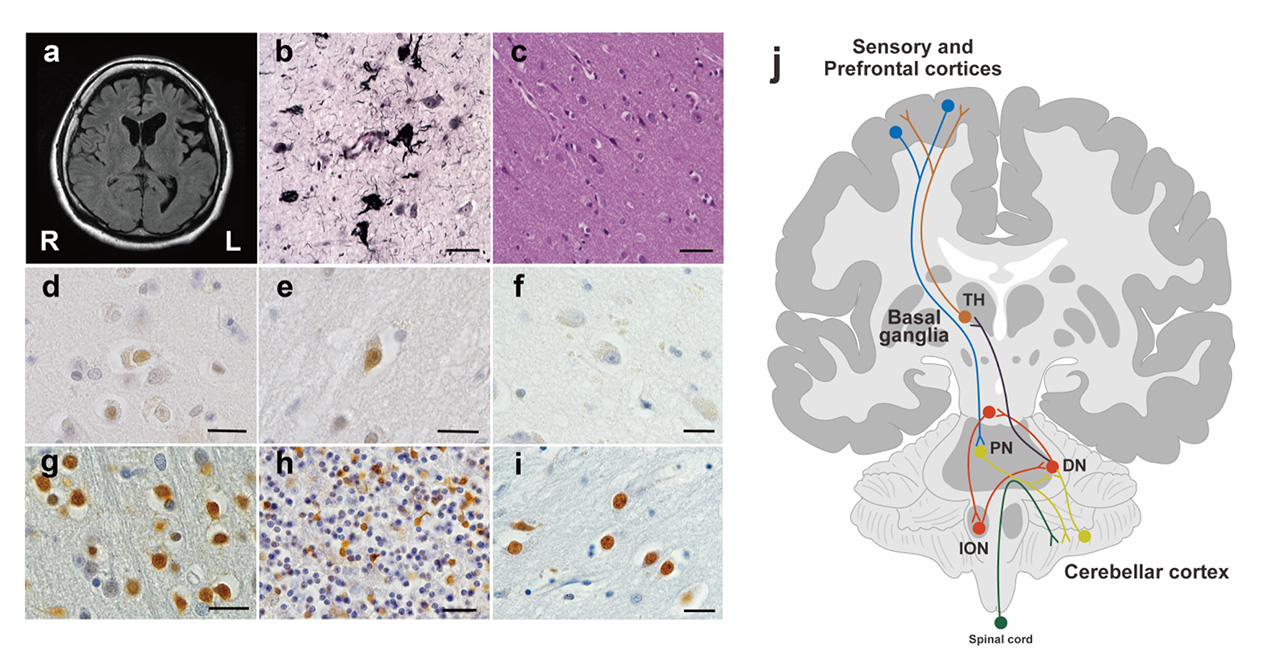
Carbonyl stress is an abnormal accumulation of reactive carbonyl species that leads to damage to proteins and increases their modification. In previous studies, we have shown that carbonyl stress increases in some patients with schizophrenia, even without complications of diabetes mellitus, a chronic kidney disease. In this subpopulation, plasma concentration of pentosidine, one of the biomarkers of advanced glycation end-products (AGEs) generated as a consequence of facilitated carbonyl stress, is correlated with a lower serum vitamin B6, a higher propensity to inpatient status, low educational status, a longer duration of hospitalization, higher doses of antipsychotic mediation, and lower processing speed scores on the Wechsler Adult Intelligence Scale. Although peripheral biomarkers have revealed an association with these clinical features, it is not yet clear whether pentosidine accumulates in the central nervous system and in which regions of the brain are sensitive to carbonyl stress. In this study, we microscopically examined the deposition of pentosidine throughout the brain of our first patient, which led to our hypothesis that carbonyl stress is enhanced in a subpopulation of schizophrenia.
Pentosidine immunoreactivities were found in both the nucleus and the cytoplasm of cells. As the previous study has shown, there is a weak pentosidine deposit in the pyramidal cells of the frontal and temporal cortices (Fig. 1d, e). However, in the hippocampal region including the transentorhinal cortex, pentosidine-positive pyramidal cells were not detected (Fig. 1f), where NFTs were deposited (Fig. 1b). Surprisingly, pentosidine deposits were abundant in granule cells in the IV granular layer within the calcarine fissure, the primary visual cortex, granule cells in the cerebellar cortex granular layer (Fig. 1g, h) and neurons in the external globus pallidus (GPe, Fig. 1i). The immunoreactivity of pentosidine in the entire brain region of the schizophrenia patient is summarized in Figure 2. In contrast, analysis of the primary visual cortex, cerebellar cortex, and globus pallidus in patients with diabetes nephropathy disease, and dementia matched for age and sex did not show accumulation of pentosidine in neurons (Figure 3).
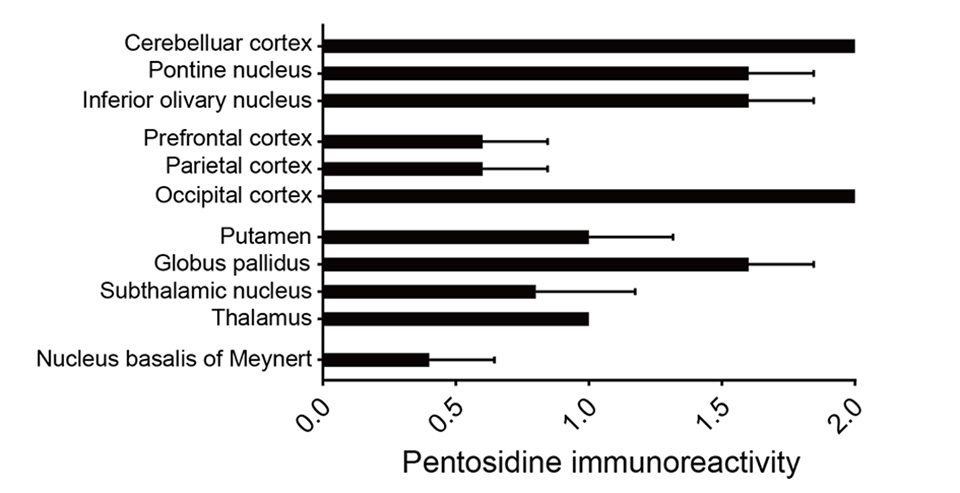
Pentosidine deposits were prominent in the cerebellar cortex, pontine, and inferior olivary nuclei. Furthermore, pentosidine deposits were found in the dorsal lateral prefrontal cortex (middle prefrontal gyrus, area 9) and in the inferior parietal cortex (supramarginal gyrus, area 40) and the primary visual cortex. In other cortical regions, the anterior cingulate cortex and the primary motor cortex, there were no pentosidine positive neurons. In the basal ganglia and thalamus, pentosidine deposits were detected in neurons of the putamen, external globus pallidus (GPe), subthalamic nucleus (STN), and thalamus. In limbic structures, only neurons in the nucleus basalis of Meynert showed weak pentosidine deposits, but there were no pentosidine positive neurons in the amygdala, the nucleus accumbens, and the locus coeruleus.
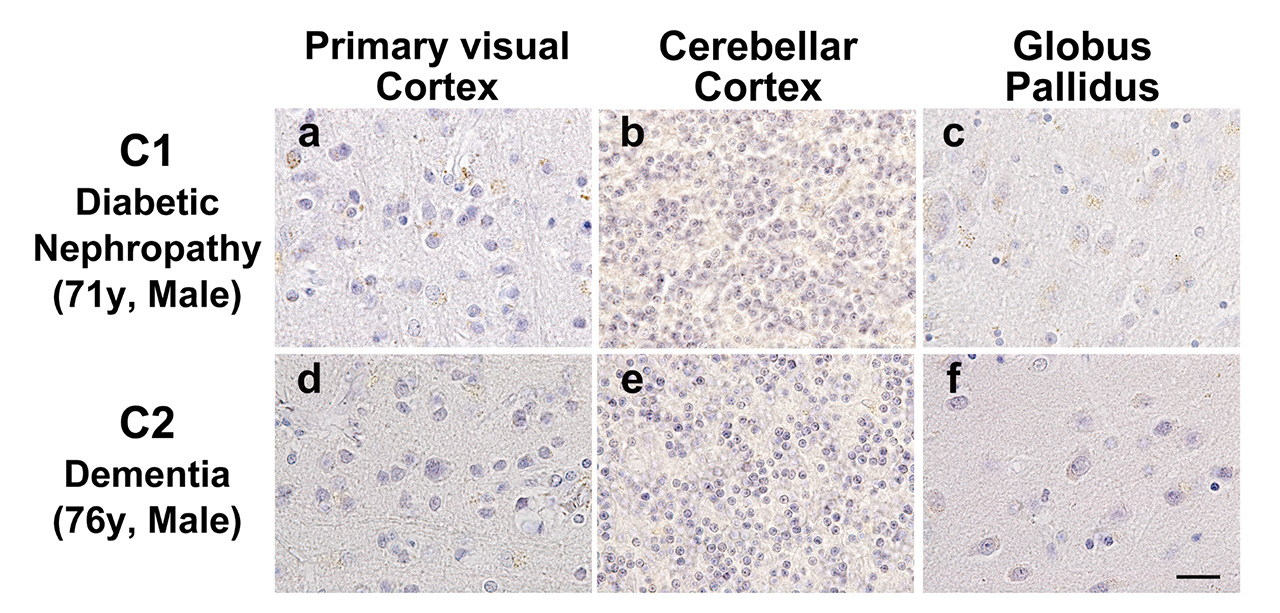
No pentosidine accumulation in (a, d) granule cells in the IV granular layer within the calcarine fissure, the primary visual cortex, (b, e) granule cells in the granular layer of the cerebellar cortex, and (c, f) neurons in the external globus pallidus in age and sex matched patients with diabetic nephropathy and dementia.
This patient with GLO1-FS is the original case that leads to our hypothesis for the subpopulation of schizophrenia with enhanced carbonyl stress. Our findings suggested that the frontoparietal cortices, auditory cortex, primary visual cortex, cerebellum, and basal ganglia could be regions sensitive to carbonyl stress in the subpopulation. More research will be required to clarify the relationship between carbonyl stress and changes in brain tissue and mental function.