
Asakura M, Toriumi K, Nozaki A, Yang J, Tatsuzaki J, Suzuki K, Miyashita M, Itokawa M, Arai M. Anthocyanins as potent inhibitors of pentosidine synthesis: Antioxidant-mediated effects. Biochem Biophys Res Commun. 2024 Dec 25;740:151007. doi:10.1016/j.bbrc.2024.151007. Epub 2024 Nov 15. PMID: 39571231.
doi:10.1016/j.bbrc.2024.151007
Advanced glycation end products (AGEs) are glycation-modified structures formed through non-enzymatic reactions between sugars and proteins or lipids. Among these, pentosidine (PEN) has been associated with aging, diabetes, Alzheimer’s disease, and other conditions [1]. Inhibiting PEN formation is considered a promising strategy for preventing or treating these diseases; however, existing AGE inhibitors have not been widely adopted due to safety concerns [2]. This underscores the urgent need for developing safer PEN inhibitors.
Our research group previously reported that a subset of schizophrenia patients exhibited significantly higher levels of PEN in their peripheral blood compared to healthy individuals [3]. Furthermore, we identified glucuronic acid (GlcA) as a precursor of PEN in schizophrenia and established an in vitro PEN synthesis system that models the molecular pathogenesis of schizophrenia [4]. Using this system, we were able to efficiently screen PEN synthesis inhibitors.
While previous studies have explored natural compounds that inhibit AGE synthesis, large-scale screening for PEN-specific inhibitors using a comprehensive compound library had not been conducted. In this study, we utilized a plant-derived natural compound library and our in vitro PEN synthesis system to identify effective PEN inhibitors. This approach has the potential to lead to novel therapeutic strategies and safer preventive treatments for PEN accumulation-related diseases, including schizophrenia.
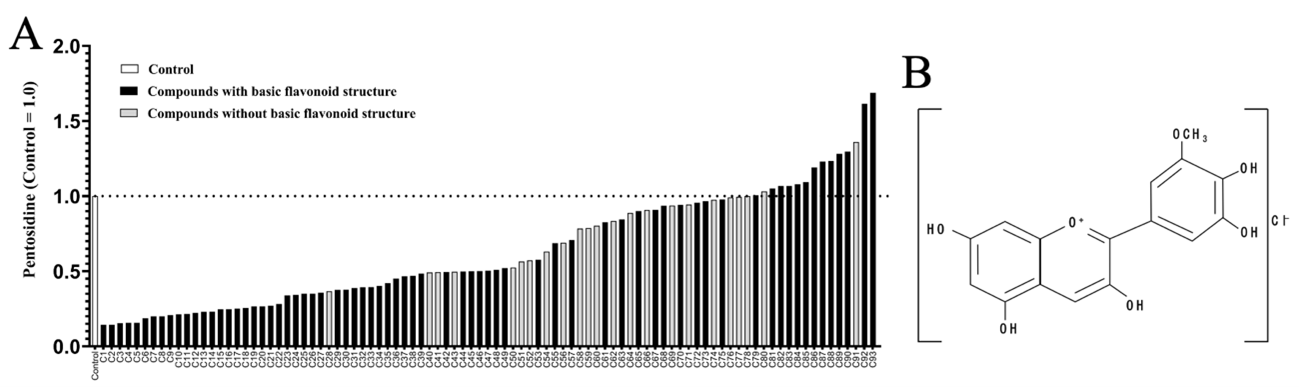
(A) PEN was synthesized in vitro by incubating human plasma with GlcA. A total of 93 natural compounds (C1–C93) were screened by adding them to the PEN synthesis system, and the amount of PEN synthesized was expressed as a relative value with the control set to 1. Compounds with a flavonoid backbone exhibited strong inhibitory activity. (red-yellow).
(B) The chemical structure of Petunidin chloride (C1), the compound with the strongest inhibitory effect, is shown.
The five compounds with the highest PEN inhibitory effects (C1–C5) were all anthocyanins. Among them, Petunidin chloride (C1) inhibited PEN synthesis by approximately 90%. A comparison of the half-maximal inhibitory concentrations (IC50) of C1 and the existing drug pyridoxamine revealed that C1’s IC50 value was about 85 times lower, indicating its potential for efficacy at much lower doses than pyridoxamine (Figure 2). These findings suggest that C1 could be a promising candidate for developing new treatments targeting PEN-related schizophrenia..
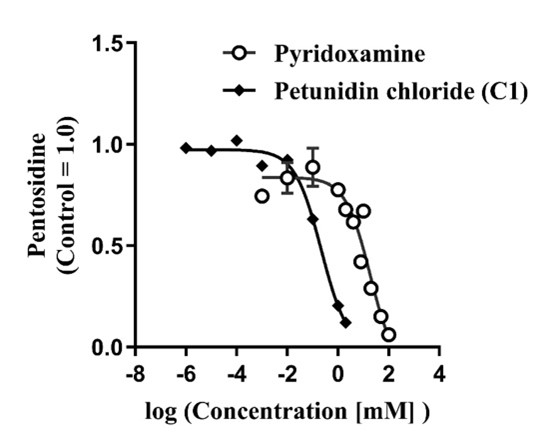
Various concentrations of Petunidin chloride (C1) or pyridoxamine were incubated with human plasma containing 2 mM GlcA. The amount of PEN synthesized after incubation was presented as a relative score compared to the control (Petunidin chloride IC50 = 0.22 mM; pyridoxamine IC50 = 18.80 mM).
Additionally, since oxidative processes play a key role in the synthesis of many AGEs, including PEN, we examined the relationship between the antioxidant activity of natural compounds and their PEN inhibitory effects. A significant correlation was observed between PEN inhibition and the oxygen radical absorbance capacity (ORAC) values of the compounds, confirming that compounds with higher antioxidant capacities tend to have stronger PEN inhibitory effects (Figure 3). This indicates that the PEN synthesis inhibitory effect may partially depend on the antioxidant properties of the compounds.
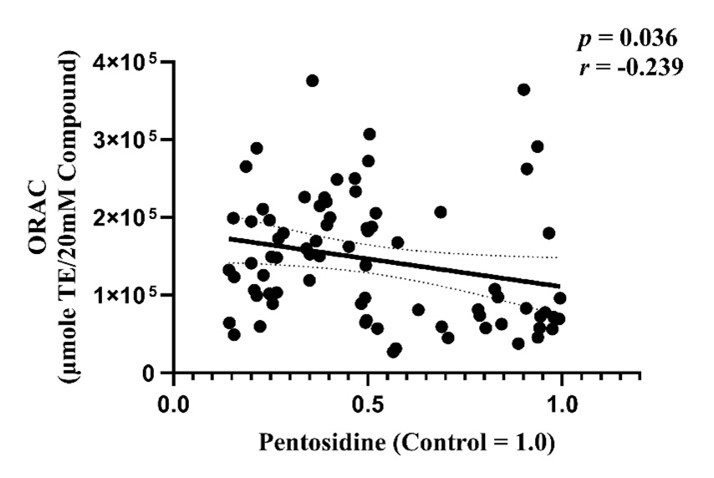
The antioxidant capacity of natural compounds (C1–C77) was quantified, and its correlation with PEN synthesis inhibition was analyzed.
These findings provide a foundation for new therapeutic strategies targeting PEN accumulation-related diseases, particularly schizophrenia, and offer valuable insights for the development of safer preventive treatments.