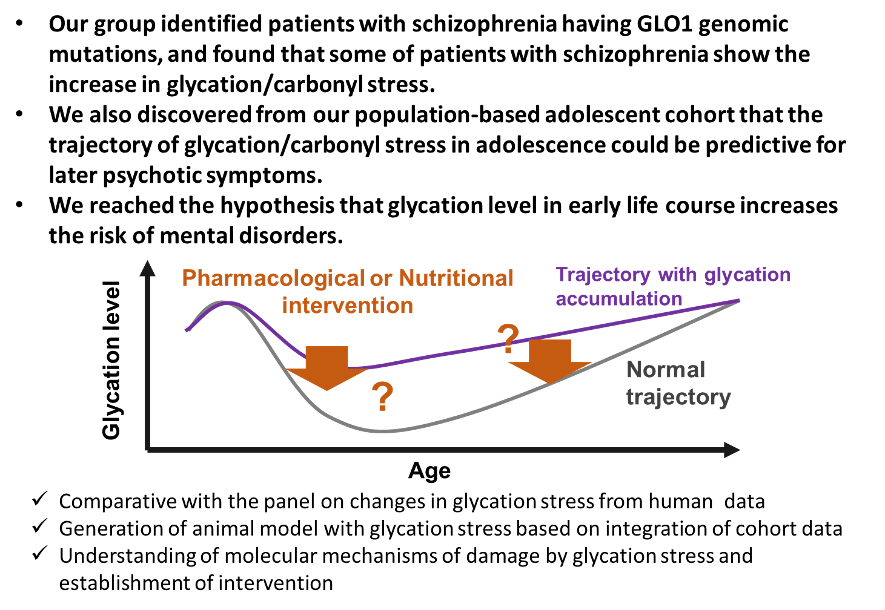
The profiling of the peripheral metabolic system is a viable schizophrenia research strategy that can lead to earlier diagnostic methods, elucidation of its molecular mechanisms, and novel strategies for the prevention and treatment. We focus on 1) developing individualized medicine for treating schizophrenia, 2) investigating factors involved in disease onset, and 3) understanding the molecular pathology by using biomarkers to overcome the barrier of heterogeneity. Our research outcomes will be applied to drug development by establishing a new biomarker-based field of research in molecular psychiatry. Data obtained from metabolomics, genomics, induced pluripotent stem (iPS) cell models, animal models, post-mortem brain analyses, neuropsychology, and genetic counseling research will be consolidated to elucidate the genetic and environmental factors relevant to psychiatric disorders such as schizophrenia.
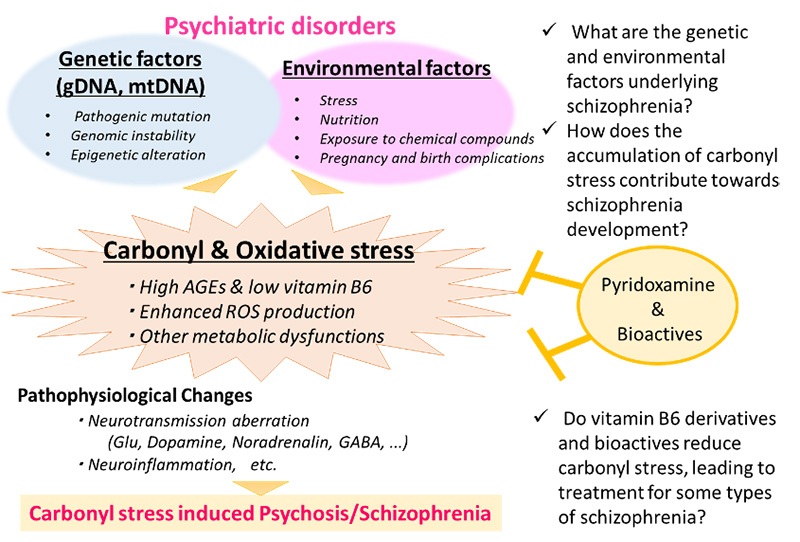
This biomarker-based approach is anticipated to become an innovative and creative strategy for elucidating the metabolic system of schizophrenia disease expression independently of conventional pathological hypotheses. Verification in cellular and animal models can shed light on the molecular mechanisms underlying the utility of naturally-derived substances, and is expected to lead to the future development of much safer treatments and prophylactic methods.

Schizophrenia Research Project,
Project Leader Makoto ARAI Ph.D
(https://www.igakuken.or.jp/english/project/
detail/schizo-dep.html)