
Ozawa A, Toriumi K, Shimada T, Endo A, Tomita T, Munesue S, Tomita Y, Takagi H, Parida IS, Miyashita M, Inagi R, Itokawa M, Yamamoto Y, Saeki Y, Arai M. Pentosidine modification of neuronal proteins induces dendritic spine enlargement in vitro. Biochem Biophys Res Commun (2025) 793: 153032.
doi: 10.1016/j.bbrc.2025.153032
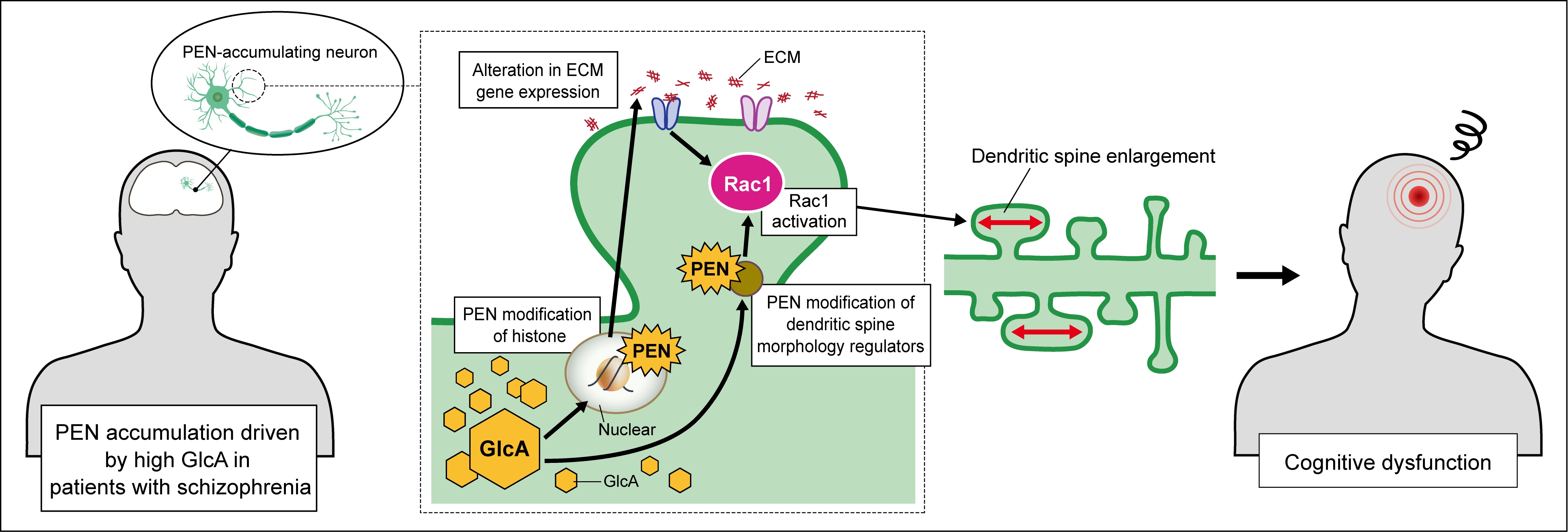
We have previously identified that pentosidine (PEN), an advanced glycation end product (AGE), accumulates in the blood of approximately 40% of patients with schizophrenia [2]. Patients with elevated PEN levels tend to show more frequent and prolonged hospitalizations and higher cumulative doses of antipsychotic medications, as well as more pronounced cognitive impairment, particularly in the domain of processing speed. In addition, these patients often present with more severe psychiatric symptoms and are more likely to be resistant to standard pharmacological treatments [3].
Glycation by AGEs is formed through the Maillard reaction under conditions of sustained hyperglycemia or metabolic stress and is known to cause loss of protein function [4]. However, the molecular mechanisms by which PEN contributes to schizophrenia pathology have remained unclear, partly because no cellular model capable of recapitulating PEN accumulation has been available.
In this study, an in vitro neuronal model that reproduces PEN accumulation within neurons was developed to investigate whether PEN-mediated protein modification disrupts neuronal structural integrity and may contribute to the pathophysiology of schizophrenia.
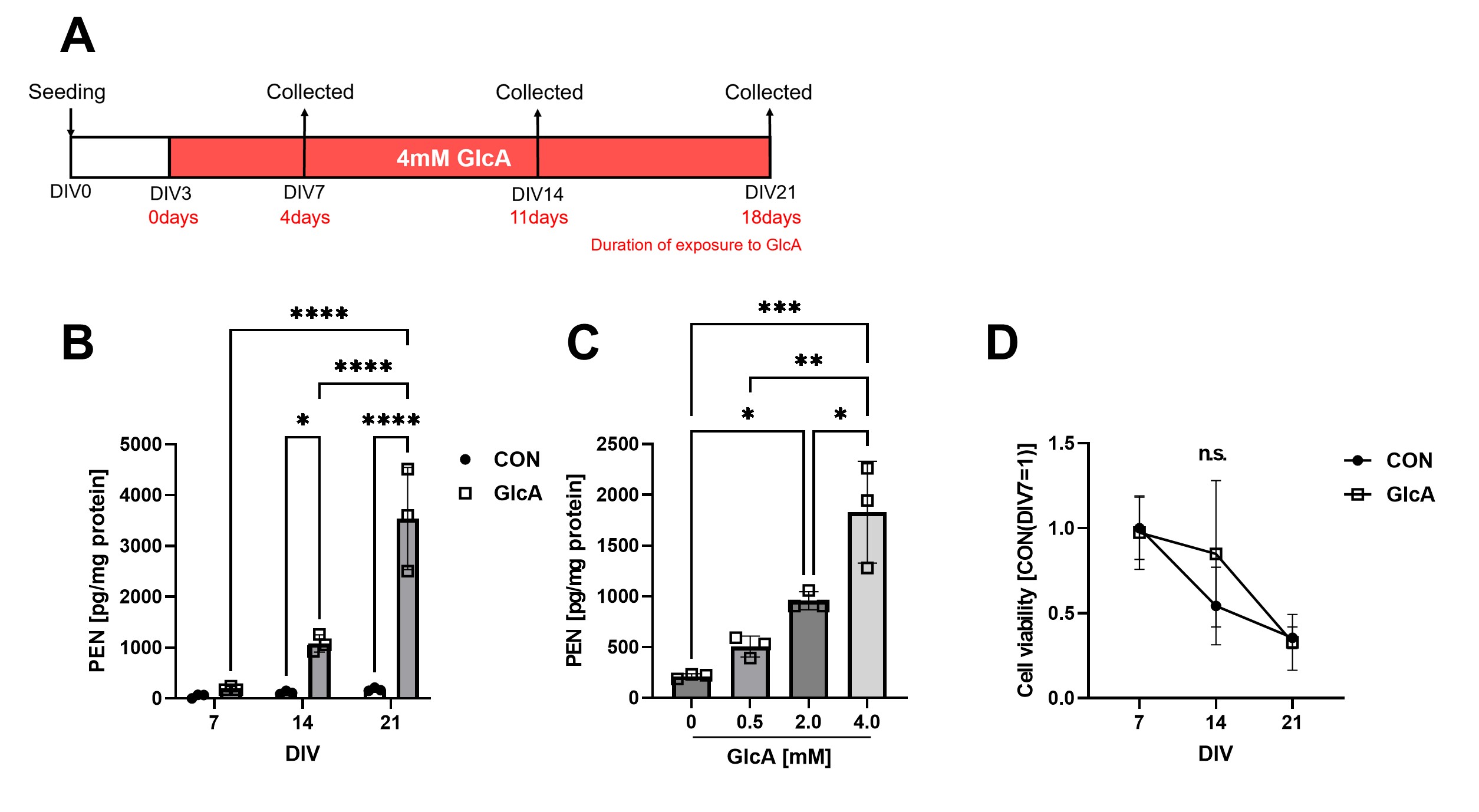
(A) Experimental timeline for generating PEN-accumulating neurons. Primary mouse cortical neurons were treated with 4 mM GlcA and maintained in culture for up to 21 days. (B) Time-course measurements showed a progressive, time-dependent increase in PEN-modified proteins following GlcA treatment. (C) PEN-modified protein levels increased in a concentration-dependent manner in response to GlcA. (D) Assessment of cell viability revealed no notable cell death in GlcA-treated neurons.
To determine which proteins undergo PEN modification, LC-MS/MS analysis was performed. Histones and regulators of Rac1 activity were among the modified proteins. Because PEN modification of histones may compete with other post-translational modifications (PTMs) and thereby alter gene expression, RNA-seq analysis was conducted. Expression changes were detected in 131 genes, and Gene Ontology enrichment analysis indicated that genes related to extracellular matrix organization were significantly affected (Fig. 2).
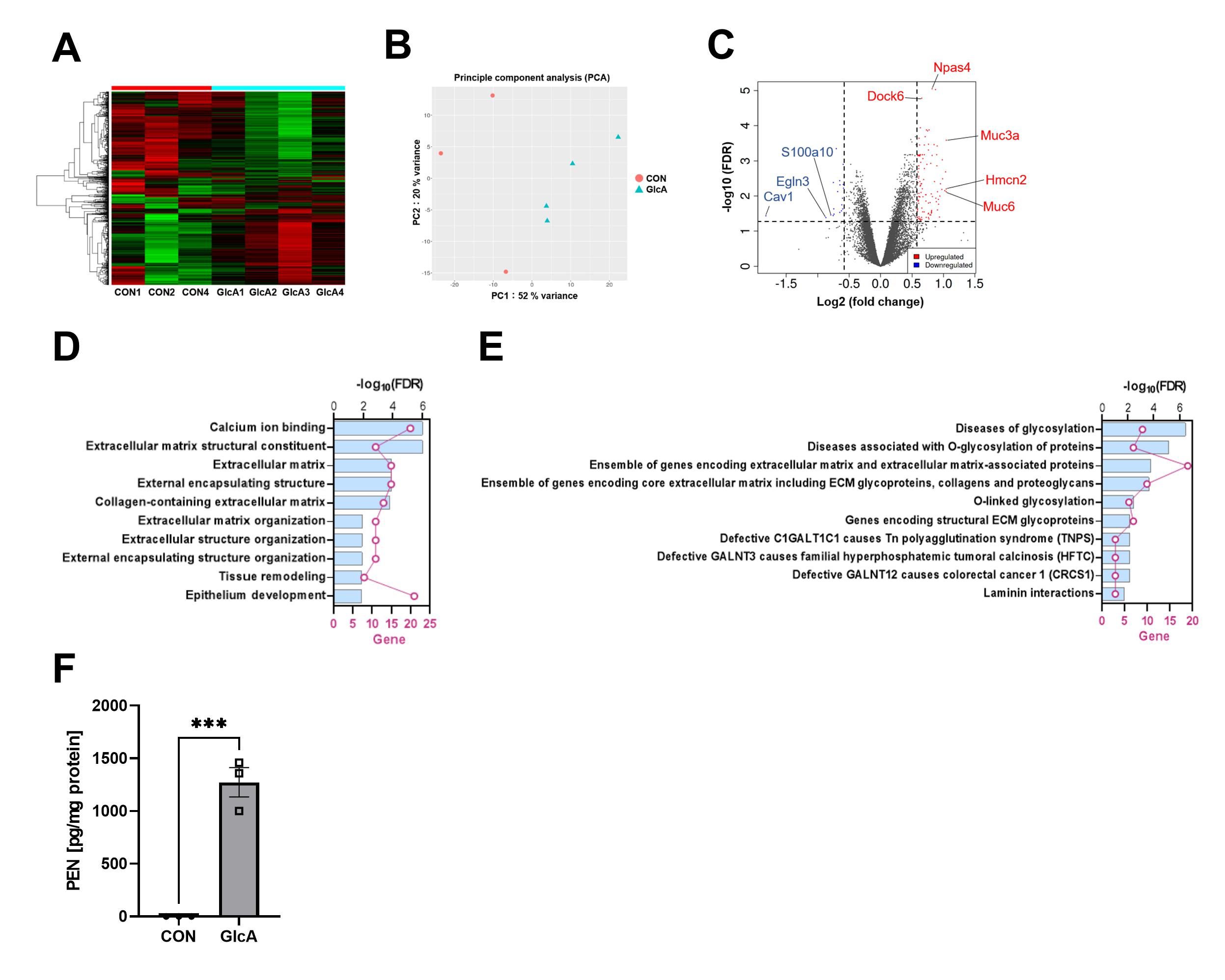
(A, B) Heatmap and PCA analyses demonstrated distinct gene expression profiles in PEN-accumulating neurons. (C) Differential expression analysis identified 112 upregulated genes and 19 downregulated genes. (D) Gene Ontology enrichment analysis of the DEGs revealed significant alterations in extracellular matrix–related genes, while (E) pathway enrichment analysis indicated altered expression of glycosylation-related genes. (F) PEN modification was also detected in histone fractions, indicating that histones undergo pentosidine modification.
Both increased extracellular matrix protein expression and altered Rac1 activity are known to influence dendritic spine morphology. Examination of spine structure revealed that PEN modification did not affect spine density or length but significantly increased spine width, particularly increasing the proportion of spines wider than 1 μm (Fig. 3).
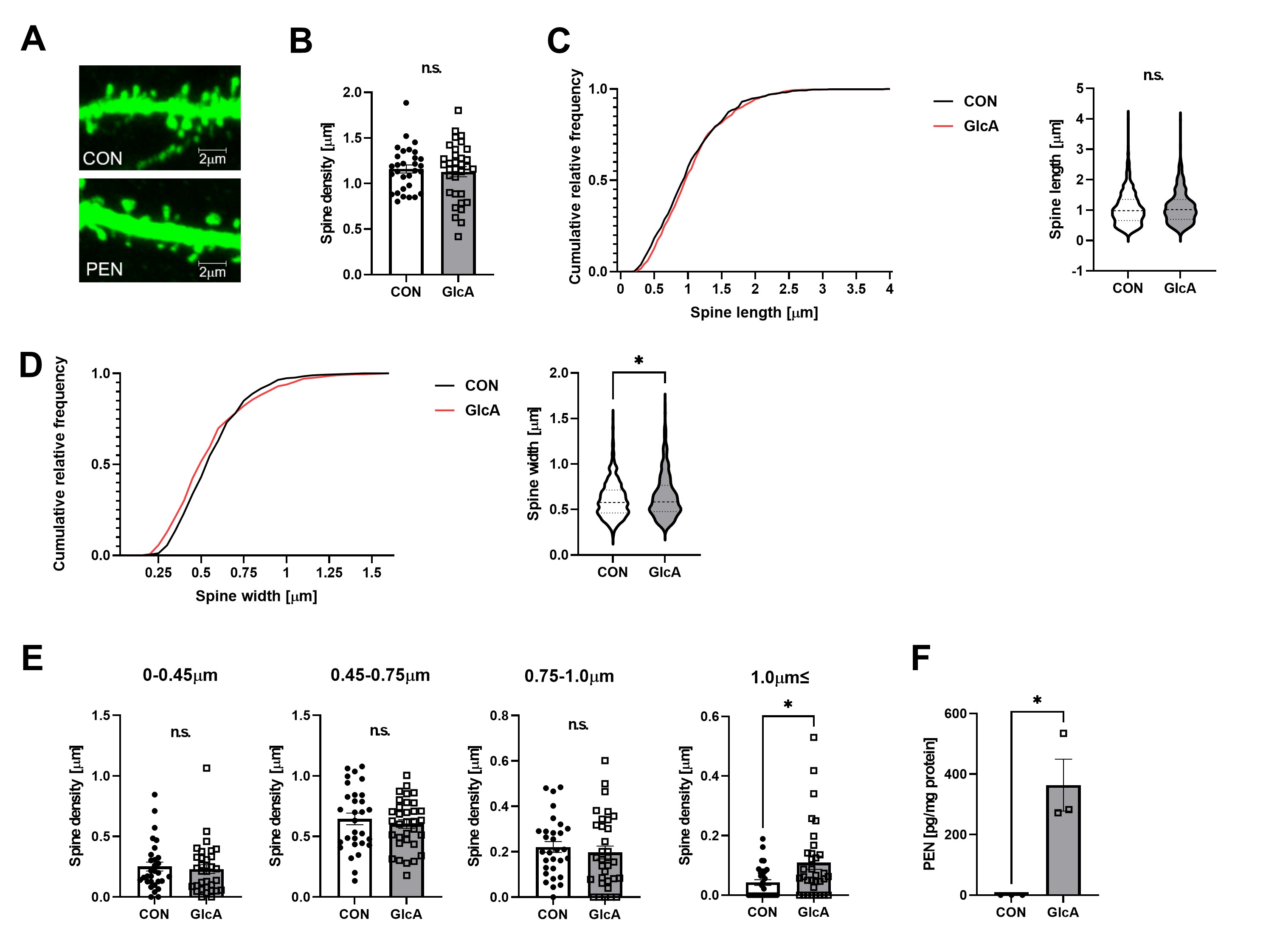
(A) Primary mouse hippocampal neurons expressing GFP were used for morphological analysis of dendritic spines. (B–D) Quantification of spine density, length, and width revealed no changes in density or length, whereas spine width was significantly increased. (E) PEN modification specifically increased the proportion of spines with widths ≥1 μm. (F) An increase in PEN-modified proteins was also observed in hippocampal neurons.
To test whether this spine enlargement was mediated by Rac1 activation, neurons were treated with the Rac1 inhibitor NSC23766. Inhibition of Rac1 prevented PEN-induced spine enlargement, demonstrating that activation of Rac1 is required for this morphological phenotype (Fig. 4). These findings indicate that PEN modification of neuronal proteins activates Rac1, leading to dendritic spine enlargement.
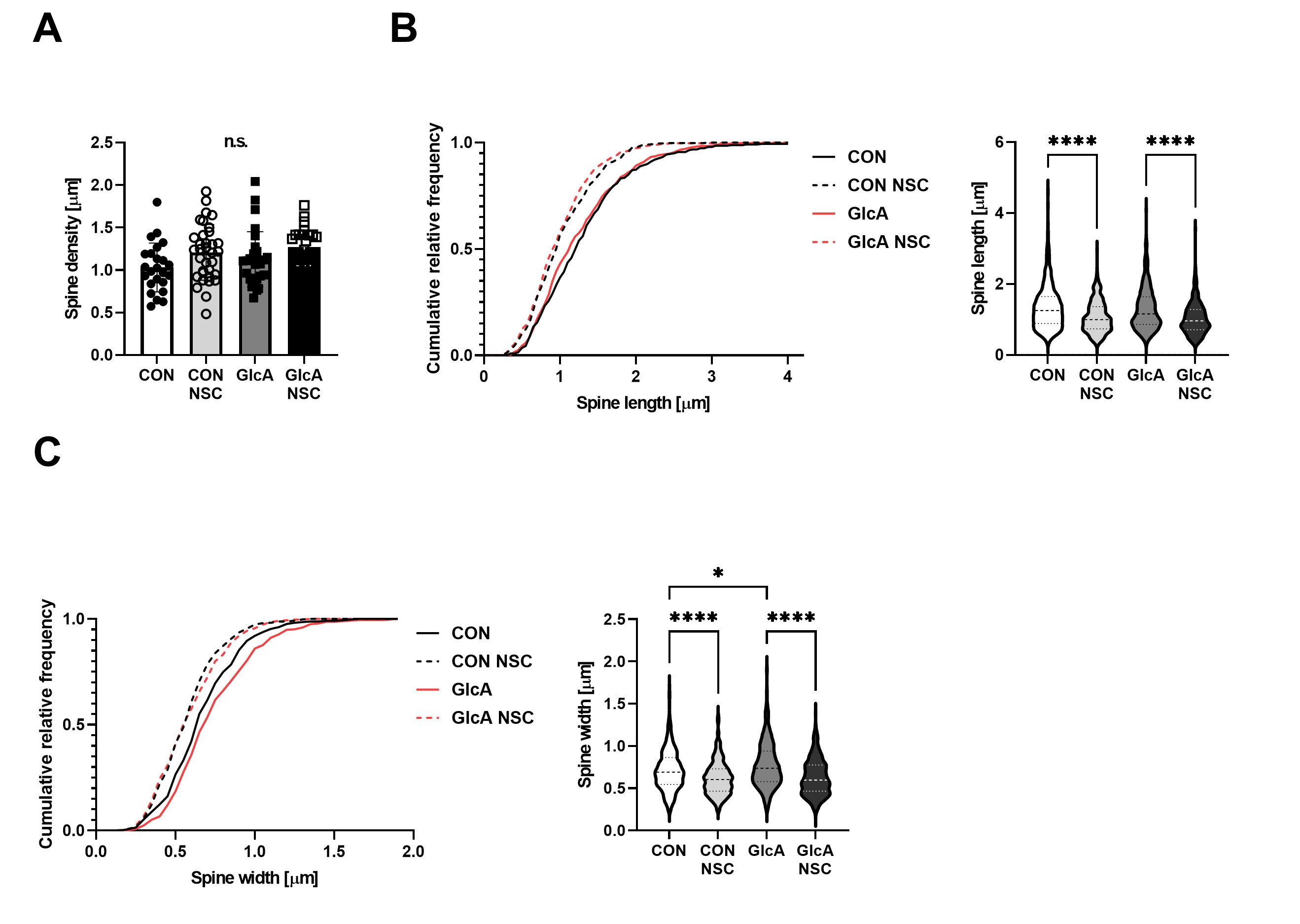
Primary mouse hippocampal neurons were treated with the Rac1 inhibitor NSC23766 (NSC), and dendritic spine (A) density, (B) length, and (C) width were analyzed. The GlcA-induced increase in spine width was normalized by NSC treatment, indicating that Rac1 activation is required for PEN-dependent spine enlargement.
Dendritic spine enlargement has recently been recognized as a pathological feature of schizophrenia. Prior studies have reported that schizophrenia risk factors increase the number of enlarged spines, enhancing neuronal firing frequency and contributing to cognitive impairment [7]. The present study suggests that PEN modification within neurons may similarly induce spine enlargement through Rac1 activation and thereby contribute to cognitive deficits in schizophrenia. This mechanism provides important insights that may inform the development of new therapeutic strategies for patients with high PEN levels who often show treatment resistance.