Meet our scientists!
 Back
Back
Our experiences get stored in our brains as memories and these experiences and corresponding mold our personalities to make us who we are. Thus, to understand who we are, we need to understand how memories are formed and retained in the brain. Tomoyuki Miyashita, in the Learning and Memory Project, is working to understand learning and memory using the fruitfly, Drosophila melanogaster. He trains Drosophila to learn to associate an odor with pain by exposing them to an odor and, at the same time, electrically shocking them. He knows that flies learn this association because they actively run away from the odor after training. Learning and memory of this association occur in a region of the fly brain called the mushroom bodies. While the neuronal pathways and neurotransmitters that transmit odor information to the mushroom bodies are known, the pathways and transmitters that convey electrical shock information have been unclear. Recently, Tomoyuki found that shock information is conveyed to the mushroom bodies through glial cells, instead of neurons, using the transmitter, glutamate. This work was published in Science in an article entitled, “Glia transmit negative valence information during aversive learning in Drosophila.” We spoke to him about this work.

What was the rationale behind this work?
In order to understand memory formation, we need to understand how pain information is conveyed to the mushroom bodies. Previously, people proposed that this information is conveyed by dopaminergic neurons, which release dopamine onto mushroom body neurons. But dopamine seems to be a more complex transmitter involved in synaptic and neuronal plasticity instead of simply conveying sensory information. More recent work from our lab indicated that glutamate may convey this information rather than dopamine. I wanted to prove this and also identify the cells that release glutamate onto mushroom body neurons. There were very few glutamatergic neurons known to synapse onto the mushroom bodies, so I started to look for unidentified cells that could release glutamate onto the mushroom bodies. Surprisingly, I found that glial cells, which have traditionally been thought of as support cells for neurons, could release glutamate. I then showed that inhibiting glutamate release from these glial cells prevented transmission of shock information to the mushroom bodies, while artificially inducing glutamate release from glia could mimic the effects of electrical shock during training.
Why was this result so surprising?
Neurons have always been thought of as the cells that rapidly transmit information throughout the nervous system. Glia were either thought of as support cells that provided neurons with energy, or as modulatory cells that allowed neurons to work more efficiently or modified the signaling of neurons by regulating neurotransmitter reuptake. Glia were not thought of as cells that directly or rapidly transmitted information. In fact, it was even debated whether vesicular release of transmitters occurred from glia. Our work identified a new aspect of glia that wasn’t previously known.
Why do you think shock information might be transmitted by glia instead of neurons?
A traditional neuronal synapse involves release of neurotransmitters from a specific presynaptic neuron to a specific post-synaptic neuron. In other words, these connections are one-to-one. A particular neuron may synapse onto many different neurons by forming many different synapses, but each synapse is one-to-one. However, there are thousands of different mushroom body neurons that respond to many different odors. Pain information needs to be sent to all of these neurons since flies can form associations between different odors and pain depending on what odor is used during training. The type of glia that releases glutamate and conveys shock information is called ensheathing glia. Ensheathing glia surround or ensheath different mushroom body compartments. My data suggest that when flies are shocked, ensheathing glia release glutamate into specific mushroom body compartments. Glutamate diffuses throughout a compartment binding to a type of glutamate receptor called NMDA receptors on all neuronal axons within a compartment. This type of volume release may be more efficient when conveying information to large numbers of neurons than traditional one-to-one synapses.
If glutamate is released onto many different neurons, how can flies learn to run away from one specific odor?
Even though glutamate binds to NMDA receptors throughout a mushroom body compartment, glutamate binding by itself isn’t able to activate NMDA receptors. NMDA receptors are called coincidence detectors because they require two different stimuli in order to become active, glutamate binding and post-synaptic depolarization. Post-synaptic depolarization occurs upon odor exposure in specific odor-responsive mushroom body neurons. So, flies learn to run away from an odor if it is paired with electrical shocks. In the brain, odor exposure causes depolarization in specific mushroom body neurons that recognize the odor. If flies are shocked at the same time, NMDA receptors become active specifically in these odor-responsive neurons. This specificity is important for forming odor-specific memories.
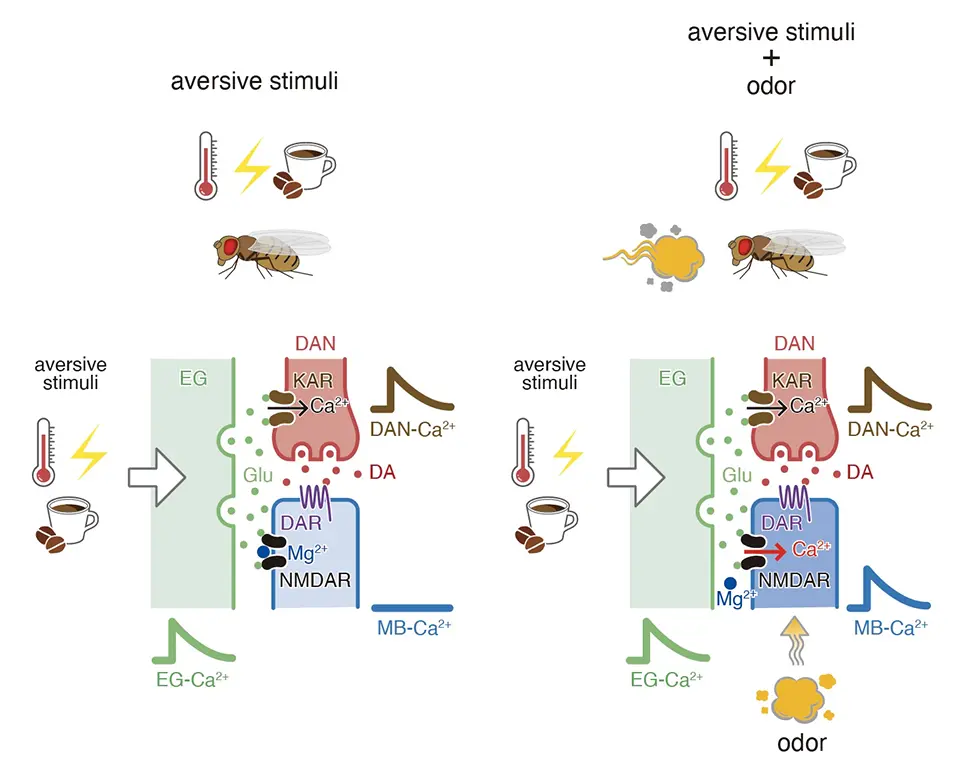
Ensheathing glia transmit aversive information.
Aversive stimuli induce vesicular exocytosis from ensheathing glia (EG) and release of glutamate (Glu) onto kainate receptors (KAR) on dopaminergic neurons (DANs) and NMDA receptors (NMDARs) on mushroom body (MB) neurons. When aversive stimuli are unpaired, Mg2+ block prevents Ca2+ influx (left). When paired with odors, odor-dependent depolarization removes Mg2+ block allowing Ca2+ influx into appropriate MB neurons (right).Interviewed by Jun Horiuch