Meet our scientists!
 Back
Back
We spend roughly one third of our lives asleep. Not only that, we also have to sleep regularly, alternating between sleep and wake cycles every day. Why do we need to sleep? That is the question that drives Akiyo Natsubori in the Sleep Disorders Project. Recently, she found that an excitatory neurotransmitter, serotonin, which is involved in arousal and awakening from sleep, also signals astrocytes, support cells in the brain, to provide fuel to neurons to generate the increased energy used during wakefulness. This work was published in iScience in an article entitled, “Serotonergic neurons control cortical neuronal intracellular energy dynamics by modulating astrocyte-neuron lactate shuttle.” We spoke to her about her work.

How did you first become interested in research?
I’ve always been interested in the human brain and consciousness. My curiosity about sleep stemmed naturally from this since sleep is a state when we lose consciousness. As a high school student, I wondered why we have sleep-wake cycles. What happens to the brain when we transit between sleep and wake states? These questions led me to pursue a career as a research scientist.
What was the rationale for your recent iScience paper?
Neurons are more active when we are awake compared to when we are asleep. That means that we use more energy when we are awake. But curiously, when I measured amounts of ATP, which is the principal energy source used in neurons, I found that amounts of ATP were higher in the cortex of awake animals compared to animals that were asleep. That means that there must be a tremendous increase in ATP production when animals awaken, which increases the amount of energy available when animals are awake. I wanted to find the mechanism behind this wake-dependent increase in ATP.
How did you do that?
There are several stimulatory neuronal systems that regulate wakefulness in animals. These systems use neurotransmitters such as noradrenalin, serotonin, histamine, or dopamine. When we activate these systems, they increase arousal in the animal and causes sleeping animals to awaken. I hypothesized that these systems may also regulate ATP production, and I tested this using serotonergic neurons. I first used optogenetics to activate serotonergic neurons in the dorsal raphe nucleus, which form extensive axonal connections to neurons in the cerebral cortex and regulate arousal and wakefulness. And I found that stimulation of these neurons increases amounts of ATP in cortical neurons. We also did the reverse experiment of inhibiting activity of these neurons using chemogenetic methods and found that less ATP was produced when these animals woke up.
Is the neural pathway that regulates ATP production the same as the pathway that regulates wakefulness?
Serotonin acts on cortical neurons to regulate wakefulness, but it acts on astrocytes, which are cells that support neurons, to regulate ATP production, so they are different pathways. We found that serotonin induces Ca2+ and cAMP surges in astrocytes and causes them to produce lactate. Lactate is an energy source that neurons use to produce ATP, and lactate produced in astrocytes is transported to neurons through an astrocyte-neuron lactate shuttle.
What are the implications of this work?
Although serotonin regulates both wakefulness and energy production, it does these through different cellular and molecular pathways. This suggests that there may be different states of wakefulness. For example, you might be awake with lots of energy available, or you might be awake with low energy. We usually think of being awake as an on/off switch. You are either awake or asleep. But our work suggests that multiple different pathways might contribute to the fully awakened state. Interestingly, it’s been shown that injecting ATP into the brains of mice models of depression rescues symptoms of depression. So it’s intriguing to consider that decreased lactate production in astrocytes might contribute to depression, and increasing activity of the astrocyte-neuron lactate shuttle might improve symptoms of depression. These are the current questions that I find interesting.
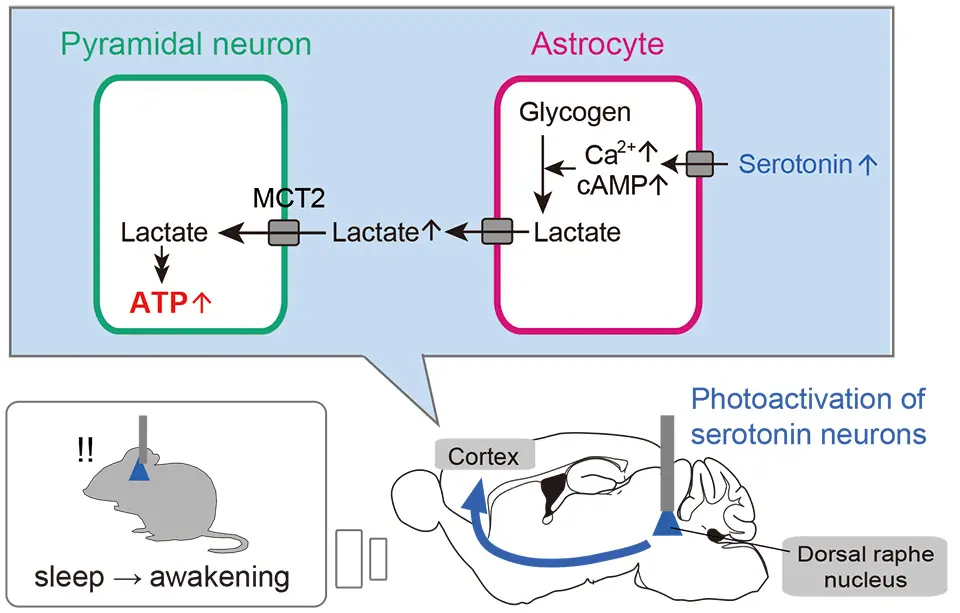
Mechanism of regulation of cerebral energy metabolism by serotonergic neurons.
The activation of serotonergic neurons in the dorsal raphe nucleus immediately awakens animals from sleep. Simultaneously, it increases intracellular ATP levels in excitatory neurons in the cerebral cortex. The mechanism involves serotonin released in the cortex acting on astrocytes, a type of glial cell, which enhances their Ca2+ and cAMP activities, promoting lactate supply from astrocytes to neurons (astrocyte-neuron lactate shuttle: ANLS). This leads to rapid intracellular ATP synthesis in cortical neurons. MCT2: A lactate transporter in neurons.Interviewed by Jun Horiuch