Meet our scientists!
 Back
Back
On July 23, 2024, we lost our Chairman Keiji Tanaka to ischemic heart disease. Dr. Tanaka was a well-loved figure in the institute. He was a pioneer and leader in the field of protein degradation, contributing extensively to the identification, cloning, and characterization of the mammalian proteasome and the ubiquitination system that targets proteins for degradation. In addition, Dr. Tanaka nurtured and mentored many further successful scientists and led TMIMS for many years, first as director, and later as chairman. Among his many protégés, Yukiko Yoshida, continues his research legacy at TMIMS by studying ubiquitination and proteasomal degradation. One of Dr. Tanaka’s contributions to proteasome research was the identification of different types of proteasomes, including immunoproteasomes and thymoproteasomes, which are required for adaptive immunity. Likewise, there are many different ubiquitination pathways besides canonical ones, and Dr. Yoshida has been studying ubiquitination mechanisms that specifically recognize and target glycosylated proteins. While most ubiquitin-conjugating enzymes recognize protein substrates, Dr. Yoshida has identified a ubiquitin ligase complex, SCFFBS2, that recognizes the sugar chains of glycosylated proteins. Further, while most proteins are modified by ubiquitination of free amino groups on proteins, Dr. Yoshida determined that SCFFBS2 can ubiquitinate a substrate glycoprotein, Nrf1, on free hydroxyl groups in a non-canonical manner. Nrf1 is important in the expression of proteasomal genes and Dr. Yoshida has shown that inactivation of Nrf1 by her identified ubiquitination pathway is responsible for symptoms of a rare genetic disorder, NGLY1 deficiency. She recently published this work (Molecular Cell 84, 3115-3127, August 22, 2024). We spoke to her about her work.

Why did you decide to become a research scientist?
It was so long ago I’ve forgotten! After graduating from college, I started working at a chemical company, and the work there was fun, but it wasn’t work where we’d discover anything new. I realized that I wanted to continue learning, so I entered graduate school and returned to academia. After graduate school and post-doc, I entered Rinshoken, which is a precursor of this institute. I studied proteins that bind to glycans, which are the sugar portion of glycoproteins. Many proteins are known to be modified by glycosylation (the attachment of sugars or sugar chains to proteins) and glycosylation is a very complicated process with more than 300 proteins functioning in forming glycans and oligosaccharides. While a lot is known about glycosylation, a lot of the functions of glycans are still unknown. At Rinshoken, I isolated glycan-binding proteins and found FBS1, 2, and 3, which work as ubiquitin ligases. Since Tanaka sensei was the foremost expert in ubiquitin, I later joined his lab and he was instrumental in helping with this work.
Tell me about Tanaka sensei.
He was subarashii (amazing, wonderful)! Now that he isn’t here, my heart is broken and it’s been difficult for me. I miss him very much. He was a great researcher, of course, but he was also very friendly and knew many people. Because he was a great scientist and a leader in the field, because he made so many friends, and because of his thirst for good science, he was also naturally a great connector. For example, I didn’t know how SCFFBS2, a ubiquitin ligase that I am studying, recognizes sugars, so he put me in touch with a famous structural biologist to figure this out. He broadened research by bringing researchers together to collaborate on interesting projects. These collaborations and connections are so vital in our field. One of the molecules that I study, NGLY1, is associated with a rare disease, NGLY1 deficiency. Tanaka sensei introduced me to a pioneer in NGLY1 research, so I’m now part of the NGLY1 deficiency rare disease community, which is very active and enthusiastic. People from many fields, about sixty researchers, are part of this community, and they’ve helped me and given me advice to speed up my research. Before, my knowledge was narrow, but now it’s so much broader.
Please tell us a little more about your work.
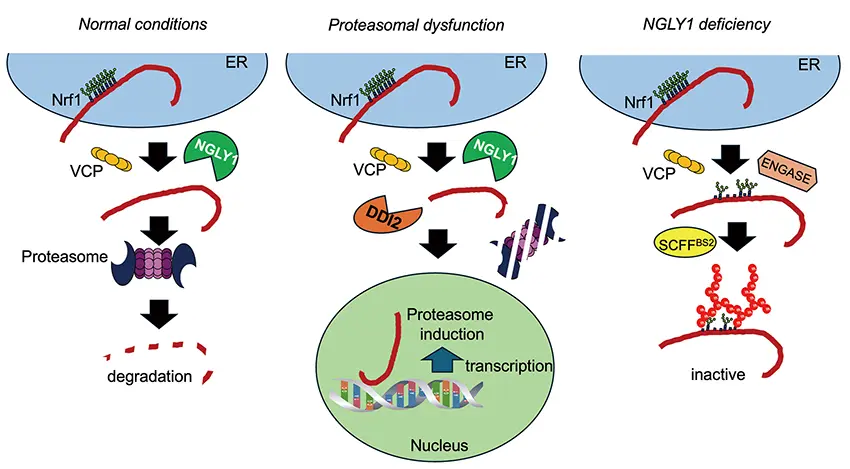
Protein degradation and recycling are critical for life, and the proteasome is required for this. So, when there aren’t enough proteasomes or when proteasomes are damaged, they need to be replaced through increased expression of proteasomal genes. This occurs through activation of a transcription factor, Nrf1, which regulates proteasomal gene expression. Under normal conditions, when cells have enough proteasomes, Nrf1 is ubiquitinated and degraded, but when cells don’t have enough proteasomes, Nrf1 doesn’t get degraded. Instead, it is cleaved by an enzyme, DDI2, and de-glycosylated by another enzyme, NGLY1. This causes Nrf1 to translocate to the nucleus where it activates proteasomal gene expression. So Nrf1 has two fates, it can either be degraded or activated. However, we found a third fate for Nrf1. When cells don’t have NGLY1, we found that the sugar molecules that are attached to Nrf1 are recognized by the sugar-dependent ubiquitin ligase, SCFFBS2, that I study. This causes Nrf1 to be ubiquitinated in a distinct way that sends it to its third fate. In this third situation, Nrf1 is neither activated nor degraded, but enters an inactive, highly deleterious state. The importance of this pathway that we’ve discovered is that it seems to be the cause of the lethality of NGLY1 deficiency.
What are your plans for the future?
Now that we know that SCFFBS2 activity is responsible for the lethality and deleterious symptoms of NGLY1 deficiency, we need to find ways of inhibiting SCFFBS2 activity. Patient groups are also eager for us to find inhibitors of FBS2. So, in collaboration with Riken, we are screening for chemical inhibitors. We hope to find something promising.
Second, we are interested in understanding the normal biological function of SCFFBS2. In our current paper, we uncovered a detailed and complex new mechanism by which glycoproteins, including Nrf1, are ubiquitinated. In “normal” or canonical ubiquitination, ubiquitin molecules are attached to the amino groups of lysine residues on proteins. However, we found that SCFFBS2 recruits an E3 ubiquitin ligase that ubiquitinates hydroxyl groups of serine and threonine residues of Nrf1 as well as a hydroxyl group on sugar molecules, GlcNAc residues, attached to Nrf1. This unconventional ubiquitination that we found is responsible for the lethality of NGLY1 deficiency, but this pathway didn’t develop just to cause disease. It must have evolved for another purpose that is beneficial to cells. We’re interested in identifying other substrates for SCFFBS2 in order to identify the normal cellular role of SCFFBS2. I want to do this for myself, of course, but I also think I owe it to Dr. Tanaka to figure this out.
Interviewed by Jun Horiuch