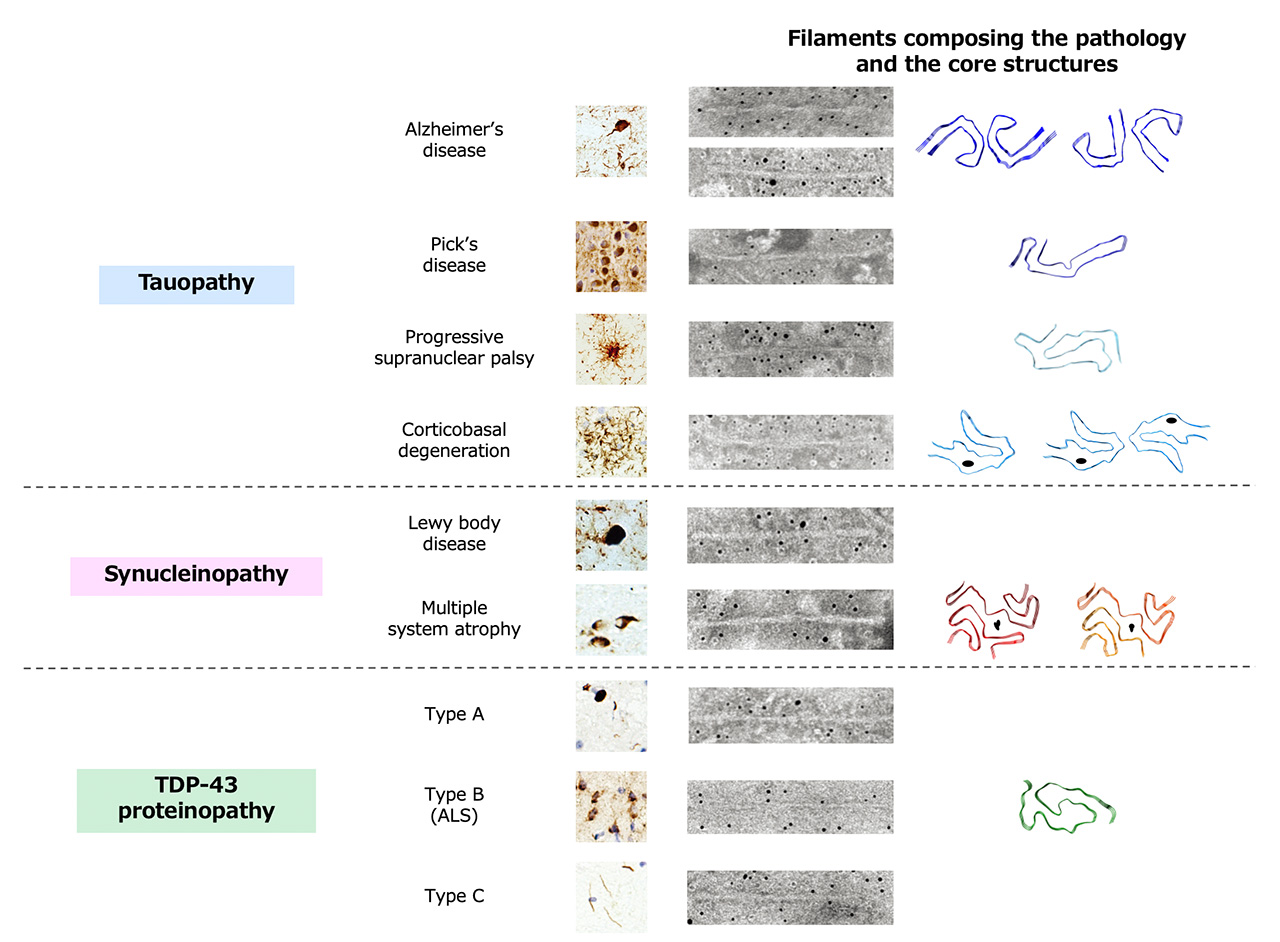
In the brains of patients with neurodegenerative diseases, abnormal proteins with conformational changes accumulate in neuronal and glial cells and cause neurodegeneration. The groups of neurodegenerative diseases in which tau, α-synuclein and TDP-43 accumulate intracellularly are referred to as tauopathies, synucleinopathies and TDP-43 proteinopathies, respectively. The clinical and pathological diversity observed in these proteinopathies is supported by the fact that abnormal proteins extracted from the patient's brain exhibit various ultrastructural and biochemical properties. Intriguingly, these ultrastructural and biochemical properties are common within the same disease group and distinguish the disease. Thus, disease classification based on these properties is not only useful for definitive diagnosis, but also suggests the presence of structural polymorphisms of abnormal proteins within the same proteinopathy. In this review, we summarized the ultrastructural and biochemical classification of pathogenic proteins that accumulate in the brains of patients with tauopathies, synucleinopathies and TDP-43 proteinopathies, including the latest insights.
Amyloid-like filaments observed in the brains of patients with neurodegenerative diseases were first described in the 1960s, and up to today the three-dimensional structures of these filaments have been revealed. This review describes the ultrastructural classification by electron microscopy and biochemical classification by immunoblot analysis of patient-derived pathogenic proteins for each proteinopathy. The core structures of patient-derived filaments identified by cryo-electron microscopic analysis are also presented. Furthermore, experimental data showing that these ultrastructural and biochemical properties are inherited in neurodegenerative disease models suggest that structural polymorphisms of abnormal proteins may have important implications in the pathogenesis that characterizes the disease. Lastly, we discuss how disease-specific abnormal protein pathology spreads throughout the patient’s brain and what factors are involved in the formation of structural polymorphisms. The ultrastructural and biochemical classification of the major neurodegenerative diseases presented in this review is expected to contribute to the development of disease-modifying agents, antibody therapies and molecular probes that will enable early therapy and diagnosis of neurodegenerative diseases.