The joint research group of Kyoto university and Tokyo Metropolitan Institute of Medical Sciences, elucidated the mechanism by which the biological rhythm of the skin controls antibacterial immunity. In order to explore the role of biological rhythms in the skin, these research groups comprehensively analyzed gene expression in mouse epidermis at various times. Then, they found that one of the CXC chemokines*1, CXCL14*2, shows fluctuation of mRNA expression according to the biological rhythm. In nocturnal mice, CXCL14 expression was high during the day and low at night, whereas in common marmosets, the same diurnal primates as humans, it was low during the day and high at night. Rhythmically expressed CXCL14 strongly binds to the DNA of Staphylococcus aureus*3 and activates innate immunity through a sensor molecule of bacterial DNA called Toll-like receptor 9 (TLR9)*4, resulting in suppression of bacterial proliferation. Thus, biological rhythms drive the activation of innate immunity at the first stage of immunity that protects the body from pathogens such as bacteria.

The skin is the outermost structure that protects individuals from the environment, and it works as the front line to protect the body from pathogens from outside the body. Organisms have a biological rhythm that synchronizes with the day-night rhythm, and the expression rhythm of the clock genes is also observed in the skin. Over the last 10 years, it has become clear that biological rhythms are involved not only in sleep-wake rhythms, but also in many physiological mechanisms such as hormones, metabolism, and immunity. However, the function of the immune system of the skin has not been clarified yet.
Okamura’s lab at Kyoto University, which has been studying the molecular mechanism of circadian rhythm, focused on the mouse epidermal tissue of the footpad, which is mainly composed of keratinocytes. They isolated footpad epidermus at various time points by using the laser microdissection method*5 and subjected them to comprehensive analysis of gene expression. Then, they found that CXCL14, one of the CXC chemokine family members, showed marked changes in expression along with biological rhythms. On the other hand, Hara’s lab at Tokyo Metropolitan Institute of Medical Science, which has been studying the function of CXCL14 with using cultured dendritic cells*6 and Cxcl14-knockout mice. The two labs have started a joint study for elucidating the role of CXCL14 in the skin.
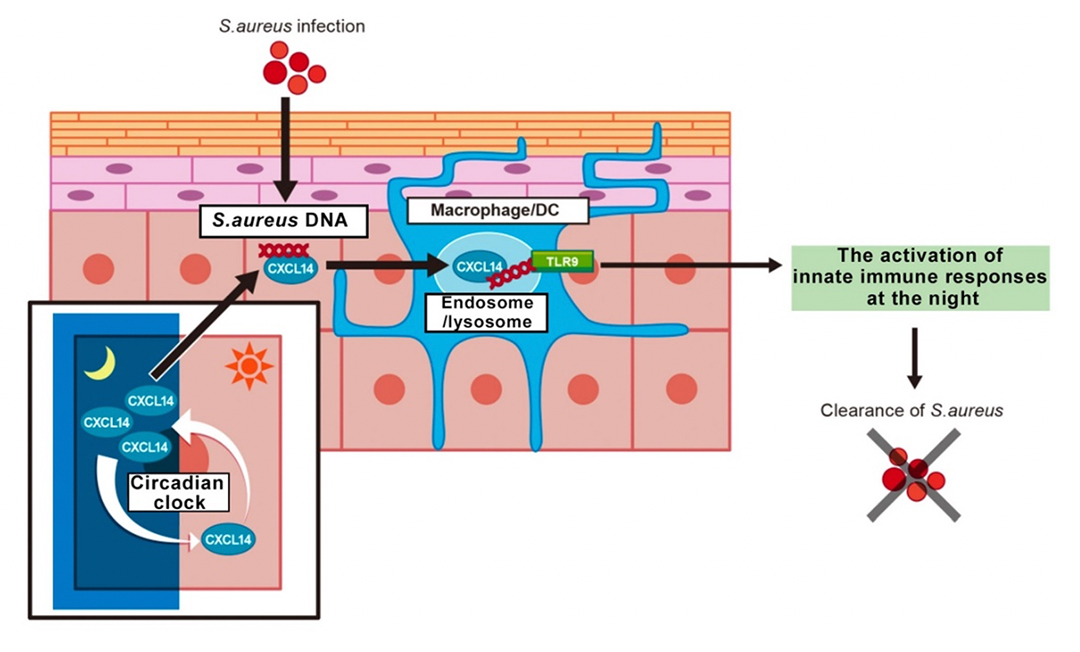
Numbers of bacteria reside on the surface of the skin. We focused on Staphylococcus aureus (S. aureus) known as a major skin-resident pathogen. We found that nocturnal mice had high expression of CXCL14 during the daytime. The growth of the bacterium was strongly suppressed in wild-type mice, but it was found that the growth of the bacterium became active at night when the expression of CXCL14 was low. In contrast, bacterial growth remained active throughout the day in the skin of Cxcl14-deficient mice.
Single-stranded DNA containing unmethylated cytosine-phosphate-guanine (CpG) motifs derived from microorganisms of bacteria or viruses is a known an activator for innate immune responses through TLR9. Consistent with previous studies of the author’s group, DNA derived from S. aureus bound to CXCL14 and increased cell surface binding on immune cells. In addition, CXCL14 enhanced the TLR9-mediated innate immune responses cooperatively with DNA from S. aureus.
CXCL14 expression in the nocturnal mouse had high daytime and low nighttime, whereas in the common marmoset, a diurnal primate, had low expression of CXCL14 in daytime and high in nighttime. We demonstrated that CXCL14 is strongly secreted from epidermal cells in the resting time, binds to the DNA of bacteria, thereby activating the TLR9 signaling pathway. Consequently, the CXCL14-TLR9 axis protects the skin from the overgrowth of S. aureus.
CXC-type chemokines such as CXCL14 have traditionally been thought to utilize CXC chemokine receptors such as CXCR4 for various cellular functions. However, CXCL14 in the skin does not utilize conventional chemokine receptors. Instead, CXCL14 binds to unmethylated CpG DNA, carries it into dendritic cells, activates TLR9 receptors within endo/lysosomes, and drives innate immunity. This cellular response is related to the function of immediate detection and elimination of foreign pathogen.
CXCL14 is widely and abundantly distributed not only in the epidermis of the skin, but also in the oral mucosa, intestinal epithelium, and respiratory epithelium. In addition, CXCL14 is expressed in many cells in the brain. It is interesting to elucidate roles and molecular mechanisms of CXCL14 in innate immune responses in these tissues.
The very strong CXCL14 rhythm in the epidermal cells of the skin is noteworthy. This rhythm was found to be produced by the clock gene product by direct transcriptional control via an RORE site in the promoter region of CXCL14 gene. Unmethylated CpG is In the future, it may be possible to enhance the antimicrobial function at the initial stage of infection by increasing the CXCL14 expression.
It is already known that biological rhythms are involved in various skin functions such as mitosis and metabolism of keratinocytes. Here we found the molecule that activate the innate immunity in the skin during the resting time. Since the CXCL14-mediated anti-bacterial defense system is strictly controlled by the circadian clock, the disruption of biological rhythm might be linked to malfunction of the health of skin
Both the biological rhythm and the innate immune system are very old biological function systems. Both of them are evolutionally conserved throughout the vertebrates, or might even earlier, when eukaryotes emerged. Thus, the both systems might have evolved together fighting the infection of bacteria. The evolutionarily conserved link between two ancient biological pathways – innate immunity and biological clocks --, which will open the door to a possible protection and treatment to the invasion of microorganisms.
This research is mainly conducted by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Scientific Research Fund (Research Project No. 15H01843; 18H04015; 18K07313; 20K08702; 20K20864; 21K07208), Ministry of Education, Culture, Sports, Science and Technology Special Promotion Research (18002016), Ministry of Education, Culture, Sports, Science and Technology (JST) CREST (It was carried out with the support of Research Project No. JPMJCR14W3).