
HOME > Topics2018 > 23 January 2018
30 January 2018
Dr. Koji Yamano, published a paper on “Elucidation of a new mechanism in which damaged mitochondria are degraded through autophagy machinery” in Elife.
Elucidation of a new mechanism in which damaged mitochondria are degraded through autophagy machinery
【Points】
- Accumulation of damaged (or dysfunctional) mitochondria in cells may cause neurodegenerative diseases such as Parkinson’s disease
- We discovered a new mechanism in which proteins involving endocytosis pathway activate autophagy and mediate degradation of damaged mitochondria.
- This study will contribute a deeper understanding of the hereditary Parkinson’s disease.
Dr. Koji Yamano, Dr. Noriyuki Matsuda, and Dr. Keiji Tanaka, researchers at the Tokyo Metropolitan Institute of Medical Science (Ubiquitin project team and Protein metabolism Laboratory) in collaboration with Dr. Richard Youle (National Institutes of Health, USA), Dr. Wade Harper (Harvard Medical School, USA), Dr. Nobuo Noda (Institute of Microbial Chemistry, Japan), Dr. Masato Kanemaki (National Institute of Genetics, Japan), Yohei Hizukuri (Kyoto University, Japan) and others revealed a new mechanism mediating degradation of the damaged mitochondria. This study shows that two different degradation pathways, endocytosis and autophagy, work cooperatively to clean unwanted subcellular components such as damaged mitochondria.
- <Title of the paper>
- Endosomal Rab cycles regulate Parkin-mediated mitophagy
- <Journal>
- Elife, 7:e31326 (32 page), January 23, 2018.
doi: 10.7554/eLife.31326.
Background
Mitochondria are one of the essential organelles for cell viability that produces cellular energy ATP. However, mitochondria also produce reactive oxygen species known as ROS, which is harmful for the cell, as a by-product, and ROS makes mitochondria dysfunctional. Since dysfunctional (or damaged) mitochondria produce more ROS, cells have to remove these damaged mitochondria selectively to keep cell homeostasis. Previous studies show that the damaged mitochondria are selectively eliminated by autophagy (mitophagy), which is one of the intracellular cleaning systems, from the cells. Otherwise the damaged mitochondria accumulate in the cell, and may cause neurodegenerative diseases including Parkinson’s disease.
Ubiquitin project team at Tokyo Metropolitan Institute of Medical Science has started from 2011 and focused on the gene products mutated in the hereditary Parkinson’s disease, and found that these gene products mediate proper and selective degradation of damaged mitochondria and suppress the onset of Parkinson’s disease.
Autophagy machinery (as you know, the Nobel prize in physiology or Medicine 2016 was awarded to Dr. Yoshinori Ohsumi for his discoveries of mechanisms for autophagy), is tightly involved in this mitochondrial degradation. However, as autophagy is a complicated process associating with dynamic membrane biogenesis, many unanswered questions especially for the selective degradation of damaged mitochondria remain to be solved.
Results and Perspectives
Dr. Yamano and his colleagues previously focused on Rab GTPase regulatory factors known as a Rab-GAP (Fis1/TBC1D15/17 complex) localized on the mitochondrial outer membrane, and found that the impairment of the Rab GTPase regulation disrupted the proper mitochondrial degradation through autophagy machinery.
Interestingly, it was known that mitochondrial Rab-GAP (TBC1D15) regulates the activation of Rab7 (mainly localizes on lysosome and controls endocytosis). Endocytosis pathway is a system delivering extracellular molecules into the cell, and many of them are transported to lysosome for their degradation. On the other hand, intracellular compartments (including damaged mitochondria) are transported to lysosome through autophagy pathway for the degradation.
We therefore observed Rab7 localization in cells defective of mitochondrial Rab-GAP by microscopy. When inducing mitophagy, Rab7, which localizes on lysosome under normal conditions, was recruited to the damaged mitochondria. Furthermore, we investigated this phenomenon more carefully using microscopy and mass spectrometry, and identified that several endocytosis proteins are also involved in the phenomenon.
The damaged mitochondria are coated by ubiquitin molecules to induce autophagic degradation. In this study, we revealed that 1) ubiquitin-binding protein called RABGEF1, which is known to function in upstream of the endocytosis pathway, is recruited to the damaged mitochondria (Fig. 1), 2) endocytic Rab cascade (RABGEF1 Rab5 MON1/CCZ1 Rab7) also function on the mitochondrial membrane to activate autophagy for mitochondrial degradation (Fig. 2).
Through this study, we discovered a new pathway for mitochondrial degradation and identified the involving proteins using cultured cells. Molecular mechanism for selective degradation of damaged mitochondria is important for human health. To apply this study for human neuronal cells, further analysis should be needed.
Fig.1
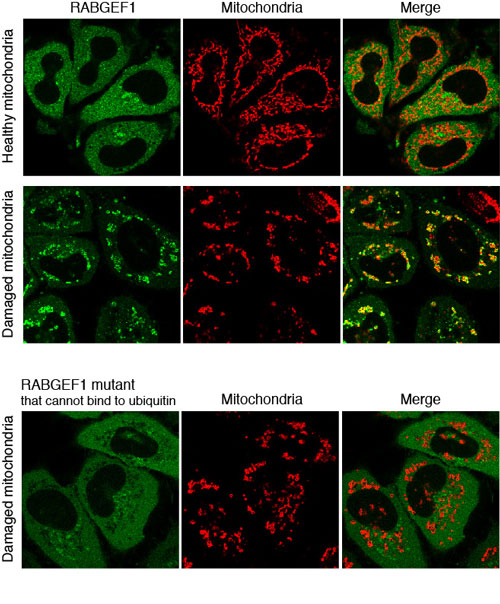
RABGEF1 is recruited to damaged mitochondria.
When mitochondria are healthy, RABGEF1 localizes in the cytosol. On the other hand, RABGEF1 is recruited to mitochondria when mitochondria get damaged. When RABGEF1 mutant that cannot bind to ubiquitin is expressed, RABGEF1 is not recruited to the damaged mitochondria, indicating that RABGEF1 recruitment is dependent on ubiquitin binding ability.
Fig.2
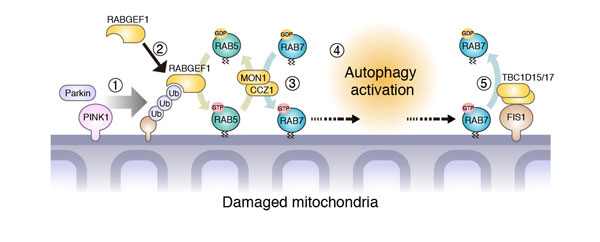
A new mechanism activating autophagy for mitochondrial degradation
①When mitochondria get damaged, Parkin and PINK1 put ubiquitin chains on the damaged mitochondria. ②Then RABGEF1 is recruited to the mitochondria, and ③ endocytic Rab and Rab-regulators (Rab5, MON1/CCZ1, and Rab7) are also recruited to the mitochondria ④ to activate autophagy. ⑤ Rab-GAP on the mitochondria (Fis1 and TBC1D15/17) facilitate the release of Rab7 from the mitochondrial membran

