Meet our scientists!
 BackTopics Page
BackTopics Page
The hepatitis C virus (HCV) is a leading cause of chronic liver disease. While effective treatments exist, up to 5% of infected people do not respond to these treatments, resulting in tens of millions of people worldwide suffering from incurable liver disease. Daisuke Yamane, a senior scientist in the Department of Diseases and Infection at TMIMS, has been studying HCV. He previously discovered a curious phenomenon. HCV viral replication is strongly inhibited by lipid peroxidation, the metabolic oxidative degradation of cellular lipids (Nature Medicine 2014 20:927-35.). In his current work, entitled “FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication,” (Cell Chemical Biology 2021 S2451-9456(21)00362-7. ) he uncovered metabolic pathways that inhibit viral replication and identified therapeutic targets for novel HCV treatments.

How did you become interested in science?
When I was in elementary school, I learned about viruses such as Ebola and HIV. I became very curious and wanted to know what they look like and how they behave in our bodies to cause disease. That was my first motivation to become a scientist and study viruses.
What do you enjoy most about research?
I enjoy discovery. I can come up with a hypothesis and I can test it. If the hypothesis turns out to be true, the findings lead to a new paper that can sometimes influence people. I just really want to know what the truth is.
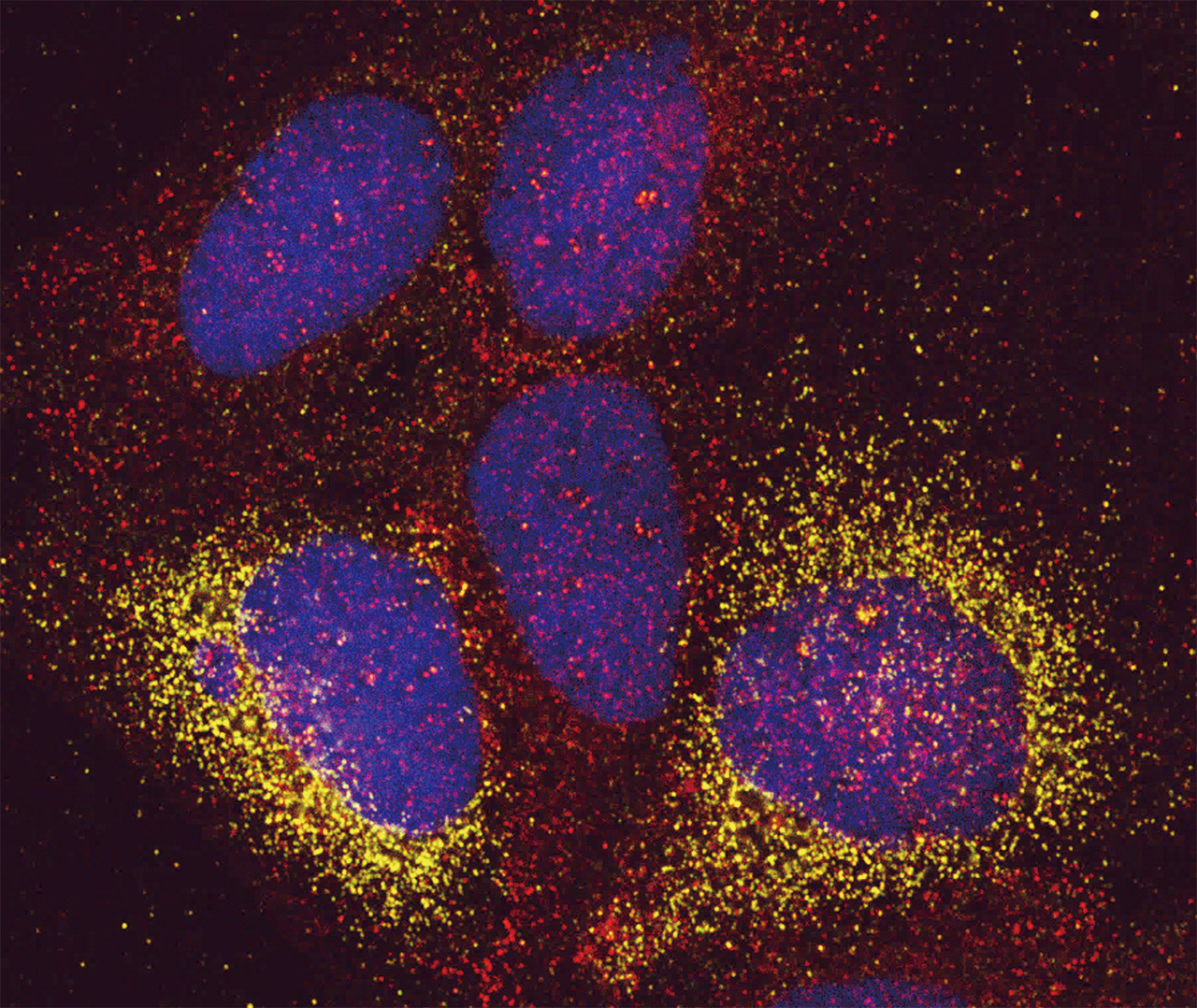
Hepatitis C virus induces remodeling of host membranes into specialized structures enriched in lipids (Red, Caveolin-2) to create viral replication factories (Green, hepatitis C virus antigen). Blue, Nucleus
How did you become interested in hepatitis C?
I went to veterinary school and first studied a bovine virus, bovine viral diarrhea virus, which is closely related to hepatitis C virus (HCV). From there, I became interested in HCV. However, HCV clinical isolates are very difficult to grow in cell culture. Their replication efficiency is very low. I tried to improve culture conditions and found that HCV is very sensitive to exogenous polyunsaturated fatty acids (PUFAs). When I added PUFAs to cell culture, it induced lipid peroxidation and this impaired viral replication. I also did the reverse experiment where I inhibited lipid peroxidation using vitamin E, a physiological lipid-soluble antioxidant. When I treated cells with vitamin E, I found that lipid peroxidation was reduced and viral replication was dramatically increased ~10 fold. From these results, I realized that cellular lipid peroxidation rates strongly influence HCV replication.
What is lipid peroxidation?
Lipid peroxidation is a process that degrades fatty acids, specifically PUFAs. PUFAs have multiple double bonds that can be attacked by free radicals. This process of attacking double bonds is amplified by chain reactions between multiple double bonds, leading to degradation of PUFAs. Lipid peroxidation is always going on at low levels in cells. Membranes have to turn over. However, when we age, lipid peroxidation increases and, in this case, it may be part of a pathological condition.
Why does replication of hepatitis C virus virus depend on the lipid metabolism state?
There are lipid peroxidation-resistant variants of HCV. These variants replicate efficiently in vitro, but in vivo,these viruses replicate too fast and are easily recognized by the immune system and eliminated. In contrast, peroxidation-sensitive viruses are more stealthy. They can infect liver cells and hide from the immune system, replicating only when the lipid peroxidation is low. They replicate gradually and slowly hijack the host cell. In fact, HCV can also induce lipid peroxidation itself. When it replicates to a threshold level, it induces lipid peroxidation which downregulates its own replication in an autoregulatory circuit. Thus, we consider that the sensitivity to lipid peroxidation may provide an advantage for the virus to persist in the host.
What is a significant finding in your Cell Chemical Biology paper?
Our paper has multiple significant findings. One is that ferroptosis inducers inhibit HCV replication. Ferroptosis is a type of cell death induced by iron. Iron acts as an oxidant and attacks the double bonds present in PUFAs to induce lipid peroxidation. We found that low concentrations of ferroptosis inducers that didn’t kill cells were sufficient to inhibit HCV replication. Furthermore, we found that ferroptosis inducers combined with direct antiviral agents that target the viral protease, a component of the viral replication machinery, exerted synergistic effects and eliminated the virus much more efficiently. Overall, we determined how hepatocytes restrict HCV replication through lipid metabolism and discovered how we may manipulate this pathway to improve therapy for hepatitis C patients.
What are your future plans?
HCV clinical isolates are still difficult to grow in culture so we are trying to develop a better cell culture system that is more similar to liver tissue in order to grow the virus more efficiently. We believe this will be important for antiviral testing and vaccine development. Despite the availability of oral drugs, HCV is still a major worldwide health concern and we think the development of vaccines is necessary for the complete elimination of HCV.

Daisuke Yamane (the first from the right on the the front row) and his former colleagues at the University of North Carolina at Chapel Hill in Prof. Stan Lemon's laboratory.