Meet our scientists!
 BackTopics Page
BackTopics Page
Inflammation can be a protective response by the body to harmful stimuli such as pathogens, tissue damage, and irritants, but it can also have adverse effects. For example, inflammation can increase the severity of various diseases, including cerebral pathologies. Koutarou Nakamura, a graduate student in the Stroke Renaissance Project, studies the inflammatory response to ischemic stroke and identified a novel signaling pathway through which strokes induce inflammation. He further found that inhibition of this pathway decreases the damage caused by strokes. We spoke to him about his recent results, published in a paper entitled, “Extracellular DJ-1 induces sterile inflammation in the ischemic brain,” PLOS Biology| https://doi.org/10.1371/journal.pbio.3000939 May 20, 2021.

How did you become interested in science?
When I was in high school, I had an excellent biology teacher who was so enthusiastic about biology that he taught us using university textbooks even though we were still in high school. In his class, I became impressed with the beauty of the immune system - how T cells, B cells, neutrophils, and many other cell types communicate with each other in a coordinated manner to protect our bodies from pathogens. When I was a third-year undergraduate student in university, I met Dr. Shichita, who was starting a new research lab at TMIMS. He invited me to join as a Master’s student. It was a great opportunity for me. Dr. Shichita’s goal was to combine the fields of immunology and neurosciences, and I was very excited to join his group to study inflammatory responses in ischemic strokes.
What are ischemic strokes?
Ischemic stroke is a brain disease where clogged blood vessels decrease cerebral blood flow. This blockage causes brain cells, including neurons, to die, leading to impaired brain functions, such as loss of movement or loss of verbal functions. One of the consequences of ischemic strokes is severe inflammation and this drastically worsens disease pathology. However, the detailed mechanisms that cause inflammation have not been sufficiently clarified, and we wanted to tackle this problem.
Why does inflammation occur?
Inflammation has both protective and harmful effects on the body. When pathogens or viruses invade our bodies, pattern recognition receptors on natural immune cells recognize these invaders. This activates natural immune cells and causes them to produce inflammatory cytokines, which protect our bodies and recruit other immune cells to the area of invasion, triggering further inflammatory immune responses. However, this inflammation also leads to severe brain edema and brain deficits after strokes. So, while inflammation has protective roles, it can also harm our bodies and worsen the pathology of diseases such as strokes.
Could you explain your results published in PLOS biology?
Even though we know that inflammation worsens the pathology of strokes, the detailed mechanisms causing inflammation were unclear. We wanted to reveal these mechanisms. In this paper, I found a molecule called DJ-1, which triggers severe inflammation after strokes and worsens stroke pathology.
Interestingly, we’ve found that DJ-1 has two opposing functions: one is cytoprotective, and the other is inflammatogenic. When DJ-1 is inside cells, it functions as a potent antioxidant that protects cells from oxidative stress and reactive oxygen species (ROSs). In ischemic strokes, reduced blood flow increases oxidative stress in neurons, leading to the production of harmful ROSs. In intact neurons, DJ-1 would catabolize ROSs, protecting cells from oxidative damage. However, we found that in more severe strokes where neurons have died from necrosis, DJ-1 is released into the extracellular space and functions as a signaling molecule to infiltrating immune cells to induce inflammation. Those endogenous molecules which induce inflammation are called DAMPs (Damage Associated Molecular Patterns) and we showed that DJ-1 is one of the major DAMP in ischemic stroke.
When we knocked out DJ-1 in mice, we observed that inflammation after ischemic stroke was strongly suppressed. However, DJ-1 knock-out mice had similar damage after stroke compared to normal mice. We think that is because DJ-1 has both protective and deleterious effects and knocking out DJ-1 removes both effects. So, we next developed neutralizing antibodies to DJ-1, which would mainly affect the harmful extracellular DJ-1. When we injected these antibodies into mice, we found that they reduced inflammation and greatly improved pathology after ischemic stroke.
What are your plans for the future?
I think that DJ-1 is a very promising therapeutic target for developing more effective treatments for stroke and other diseases associated with inflammation. Also, although DJ-1 antibodies are expensive, they could be further developed for treatments of inflammation. For myself, I’m currently interested in studying endogenous repair mechanisms in the brain. Usually the brain isn’t thought of as a very repair-friendly organ, but I’ve recently obtained some interesting results that I’m eager to pursue further.
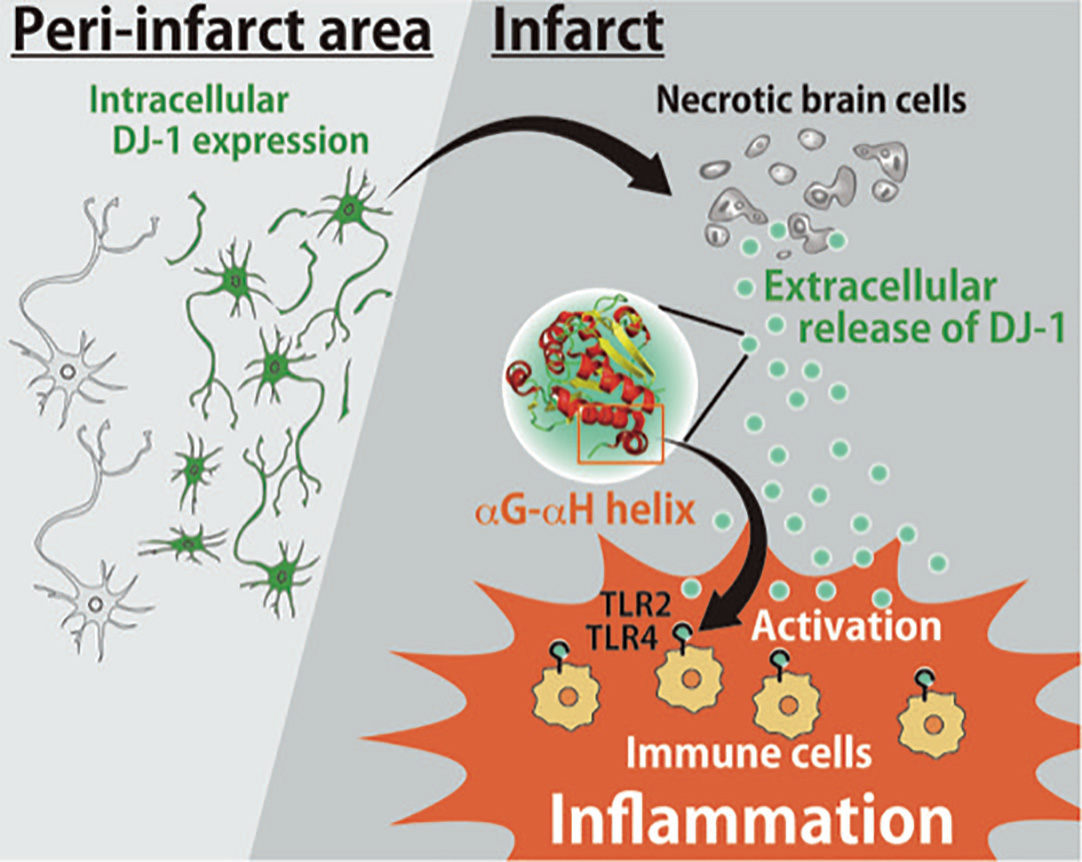
Schematic model of the roles of DJ-1 in the ischemic brain injury. DAMP, damage-associated molecular pattern; ROS, reactive oxygen species.