Mr. Koutarou Nakamura, a graduate student of Stroke Renaissance Project and Graduate School of Frontier Sciences, The University of Tokyo, has identified DJ-1 protein as the previously unknown inflammatogenic molecule which triggers sterile inflammation after ischemic stroke.
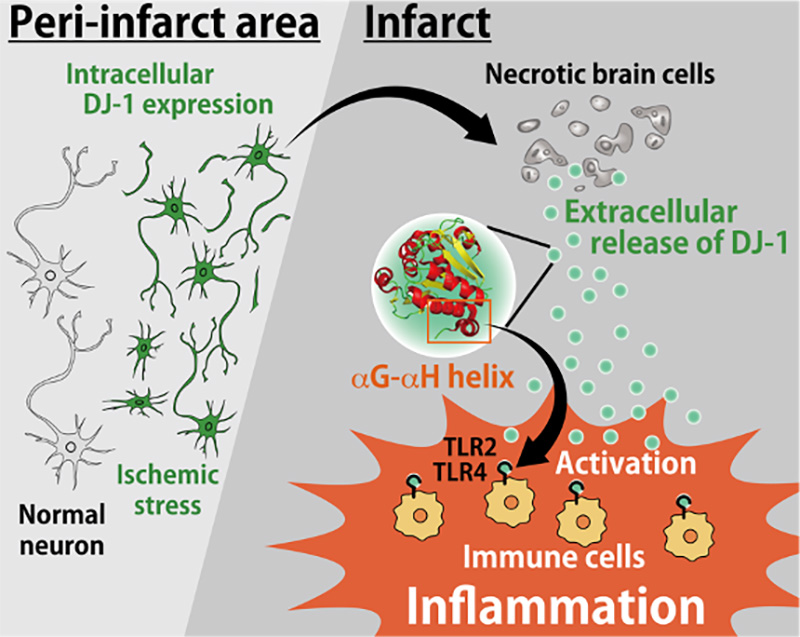
Inflammation after ischemic stroke is an important therapeutic target since cerebral inflammation leads to brain edema and further neuronal damages which worsen the functional prognosis of stroke patients. However, the molecular mechanisms underlying the trigger of cerebral post-ischemic inflammation have not been sufficiently clarified. In this study, Nakamura, et al. investigated the various proteins included in the brain lysates and identified a DJ-1 protein as the previously unknown molecule which activates immune cells to trigger the inflammation after brain tissue injury.
DJ-1 has been originally known as a neuroprotective antioxidant, whose expression increases within neurons against the ischemic stresses after stroke onset. Whereas we discovered, if ischemic stress results in neuronal cell death, the accumulated DJ-1 within ischemic neurons is released into the extracellular space. The αG–αH helix structure of DJ-1 protein released extracellularly activates infiltrating immune cells by binding to their Toll-like receptors which trigger the production of various inflammatory factors. DJ-1 thus has two opposing functions: extracellularly (inflammatogenic molecule released from dead cells) and intracellularly (neuroprotective antioxidant activity) in the pathology of ischemic stroke. We also developed the antibody to neutralize extracellular DJ-1 which had therapeutic effects against ischemic brain injury.

Ischemic stroke, which is a major cause of death and disability around the world, is the sudden onset of neurological deficits due to necrotic cell death of brain tissue caused by a severe reduction of cerebral blood flow. However current medical treatments are very limited to the acute phase of stroke. The research group has been trying to develop a long-term effective treatment after the onset of ischemic stroke by focusing on "sterile inflammation" which sustains several days after the onset and worsens the neurological deficits associated with stroke. Sterile inflammation after the stroke onset is induced by endogenous ‘self’ molecules which are released into the extracellular space from damaged tissue and activate infiltrating immune cells. These endogenous molecules are known as damage-associated molecular patterns (DAMPs).
Previously, our research group had identified peroxiredoxins (PRXs) from brain lysates, which induce the expression of inflammatory cytokines in cultured myeloid cells, as DAMPs (Shichita et al., 2012). This induction is partially reduced by the depletion of PRX proteins in brain lysates. In this study, we searched for previously unknown DAMPs in brain homogenates. We identified DJ-1 (also known as PARK7) as a major DAMP with a unique peptide sequence that activates Toll-like receptors (TLRs) in infiltrating immune cells.
DJ-1 has been thoroughly investigated as a cytoprotective antioxidant protein in neurons. However, here we demonstrate that extracellularly released DJ-1 triggers neurotoxic inflammation after ischemic stroke. Intracellular DJ-1 increases in response to oxidative stress in ischemic neurons, but if ischemic stresses result in necrotic cell death, DJ-1 is passively released into the extracellular space. This extracellular DJ-1 was in direct contact with TLR2 and TLR4 in infiltrated immune cells. We further identified a unique peptide sequence in the αG and αH helices of DJ-1 that activates TLR2 and TLR4 in infiltrated immune cells to induce the expression of inflammatory cytokines. Although DJ-1 deficiency in a murine model of middle cerebral artery occlusion did not attenuate neuronal injury, the inflammatory cytokine expression in infiltrating immune cells was significantly decreased. Next, we found that the administration of an antibody to neutralize extracellular DJ-1 suppressed cerebral post-ischemic inflammation and attenuated ischemic neuronal damage. Our results demonstrate a previously unknown function of DJ-1 as a DAMP and suggest that extracellular DJ-1 could be a therapeutic target to prevent inflammation in tissue injuries and neurodegenerative diseases.
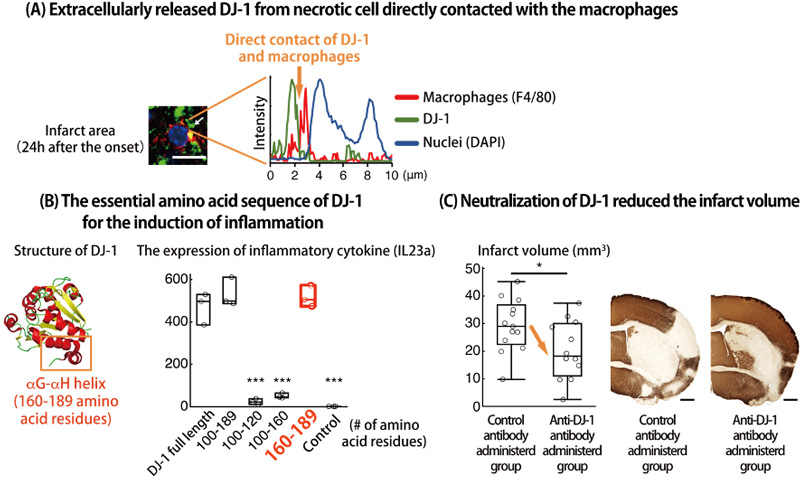
(A) Immunohistochemistry in the infarct region 24 h after stroke onset. The white arrow indicates the direct contact of DJ-1-including debris with the cellular membranes of infiltrating myeloid cells. Fluorescence intensity along the white arrow is shown in the right panel. (B) Left panel: αG helix (DJ-1160–173: peptides between residues 160 and 173 of DJ-1) and αH helix (DJ-1175–189) are indicated in the crystal structure of DJ-1 protein (PDB ID: 1P5F). Right panel: IL-23p19 (IL23a)-inducing activities of GST-fused peptides containing the C-terminal region of DJ-1 when these peptides were added to the cultures macrophages. The number of amino acid residues contained in each GST-fused DJ-1 peptide is shown on the x-axis. ***p < 0.001 vs. BMMs treated with full length of DJ-1 (one-way ANOVA) (C) Infarct volume of mice treated with the indicated antibody on day 7 after stroke onset. *p < 0.05 vs. mice treated with control IgG antibody (Student’s t-test).
###
This work was conducted by researchers in TMIMS (Tokyo Metropolitan Institute of Medical Science), Japan.
This work was supported by JSPS KAKENHI Grant-in-Aid for JSPS Research Fellow JP20J21472, PRIME from AMED under grant number JP20gm5910023, CREST from AMED under grant number JP20gm1210010, A Grant-in-Aid for Scientific Research on Innovative Areas (Dynamic regulation of brain function by the Scrap & Build system) (JP19H04765), and (Inflammation Cellular Sociology) (JP 20H04957) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), JSPS KAKENHI Grants-in-Aid for Young Scientists JP 17H05096, JP 18K14831, JP 17K15204, Toray Science and Technology Grant, Takeda Science Foundation, Mitsubishi Foundation, SENSHIN Medical Research Foundation, MSD Life Science Foundation, Senri Life Science Foundation, and Ono Medical Research Foundation.