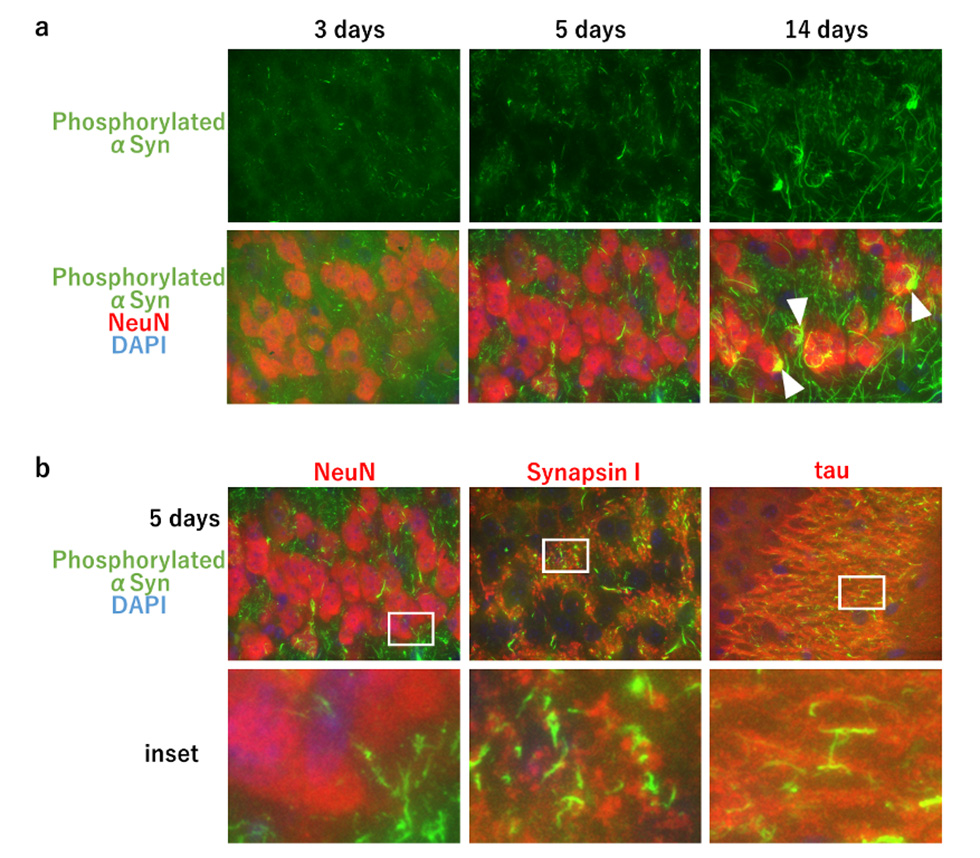
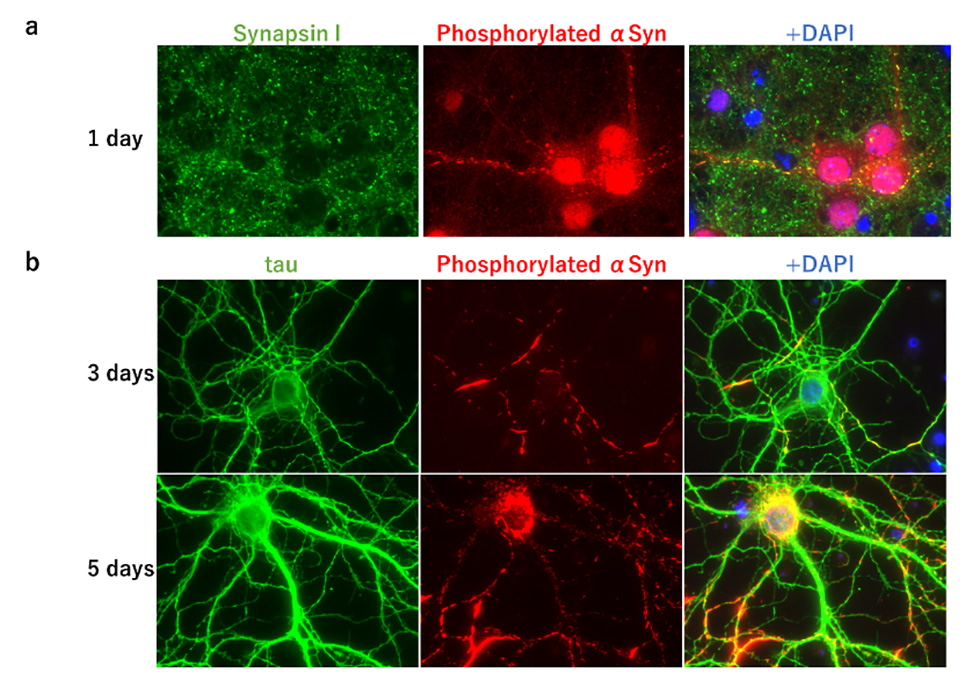
In Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy, accumulation of aggregates of abnormally phosphorylated α-synuclein is observed, and these diseases are collectively called α-synucleinopathies. It has been reported that when α-synuclein aggregates formed in vitro are injected into the brain of wild-type mice, seed-dependent phosphorylated α-synuclein aggregates are formed about one month later. But little was known about when and from where phosphorylated α-synuclein accumulation is induced after α-synuclein aggregates are injected into the mouse brain. To clarify this, we injected α-synuclein aggregates formed in vitro into the hippocampus of wild-type mice and examined the appearance of phosphorylated α-synuclein at 3, 5, and 14 days after injection. We found that dot-like forms of phosphorylated α-synuclein already appeared 3 days after injection, followed by process-like forms of phosphorylated α-synuclein 5 days later, and aggregated forms of phosphorylated α-synuclein in neuronal cell bodies 14 days later (Fig. 1a). We co-stained with marker proteins of synapses, axons, and neuronal cell bodies and found that phosphorylated α-synuclein at 5 days post-injection localized to synapses and axons, but not to neuronal cell bodies (Fig. 1b). In addition, accumulation of phosphorylated α-synuclein in the neuronal cell bodies was observed in the regions of neural communication with the hippocampus, the site of inoculation, while little accumulation of phosphorylated α-synuclein was observed at the synapse in such regions. These findings suggest that phosphorylated α-synuclein first appears at the synapse in the site of inoculation and is transported through the axon to the neuronal cell body where it forms aggregates. In addition, when α-synuclein aggregates were added to primary cultured cells prepared from fetal mouse brain, dot-like phosphorylated α-synuclein appeared at synapses after 1 day, extended its localization to axons after 3 days, and became aggregates in neuronal cell bodies after 7 days (Fig. 2). Taken together, these results indicate that in the presence of seed, phosphorylated α-synuclein begins to emerge from neuronal synapses, undergoes localization expansion on axons, and forms phosphorylated α-synuclein aggregates in neuronal cell bodies. Our results show that seed-dependent phosphorylation of α-synuclein begins in the synaptic region. This suggests that seeded α-synuclein aggregates may be taken up from synapses, which may provide an insight into the uptake of α-synuclein aggregates into neurons, an important process in prion-like propagation.