Apoptosis signal-related kinase 1 (ASK1) has been shown to cause neuroinflammation in neurodegenerative disease models, but its mechanism of action has been unclear. Xiaoli Guo and her colleagues at Visual Research Project demonstrated that ASK1 is required in microglia and astrocytes to cause and maintain neuroinflammation by a feedback loop between these two cell types during neuroinflammation in a disease model of multiple sclerosis (MS).

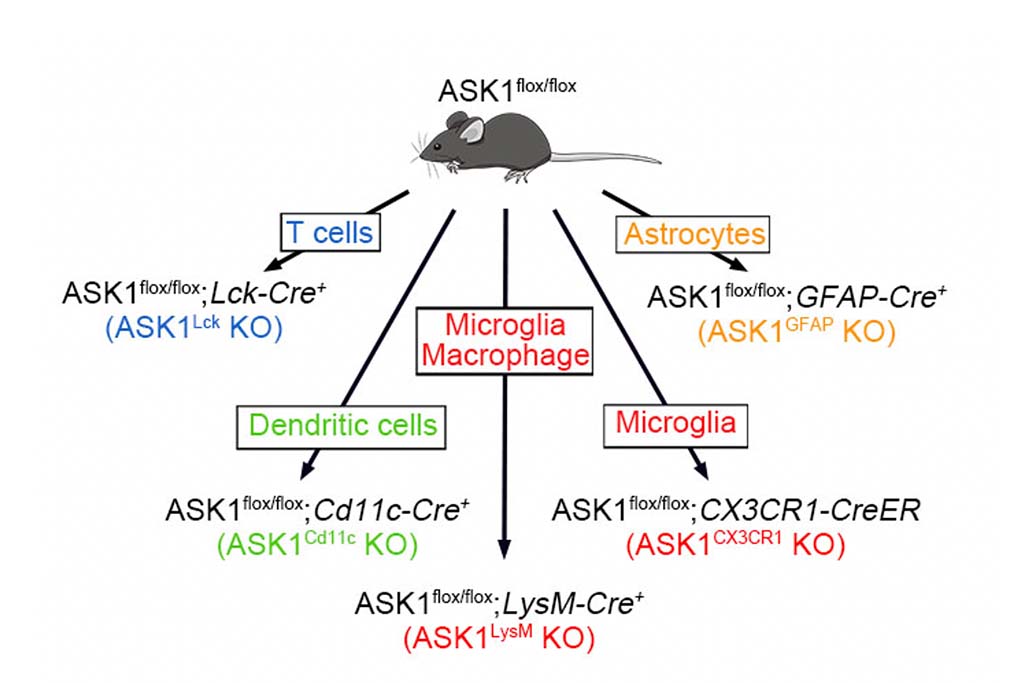
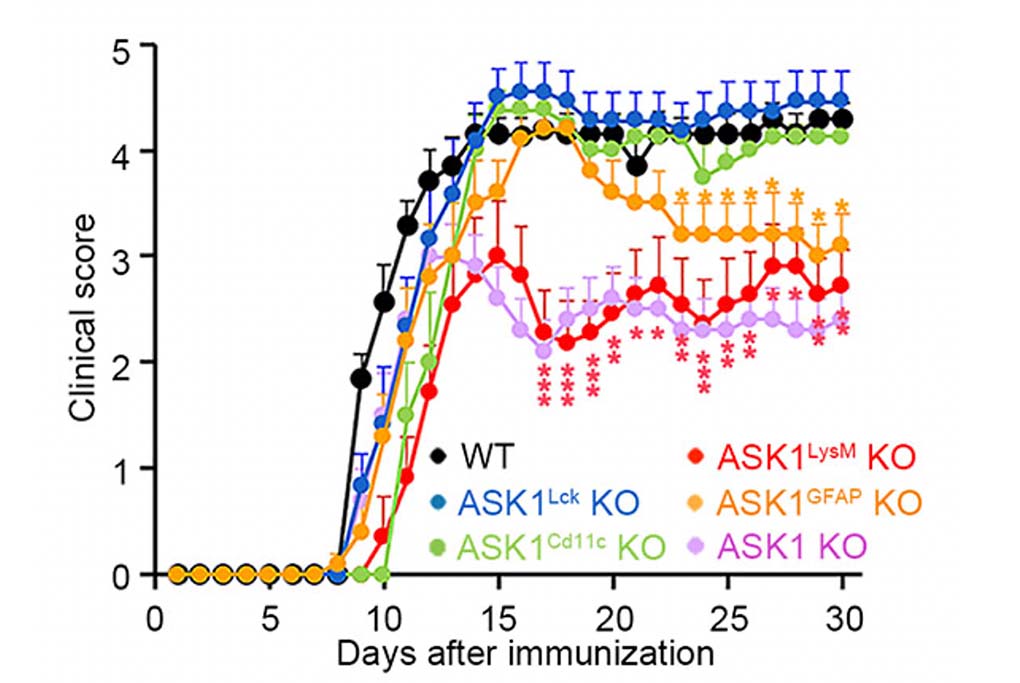
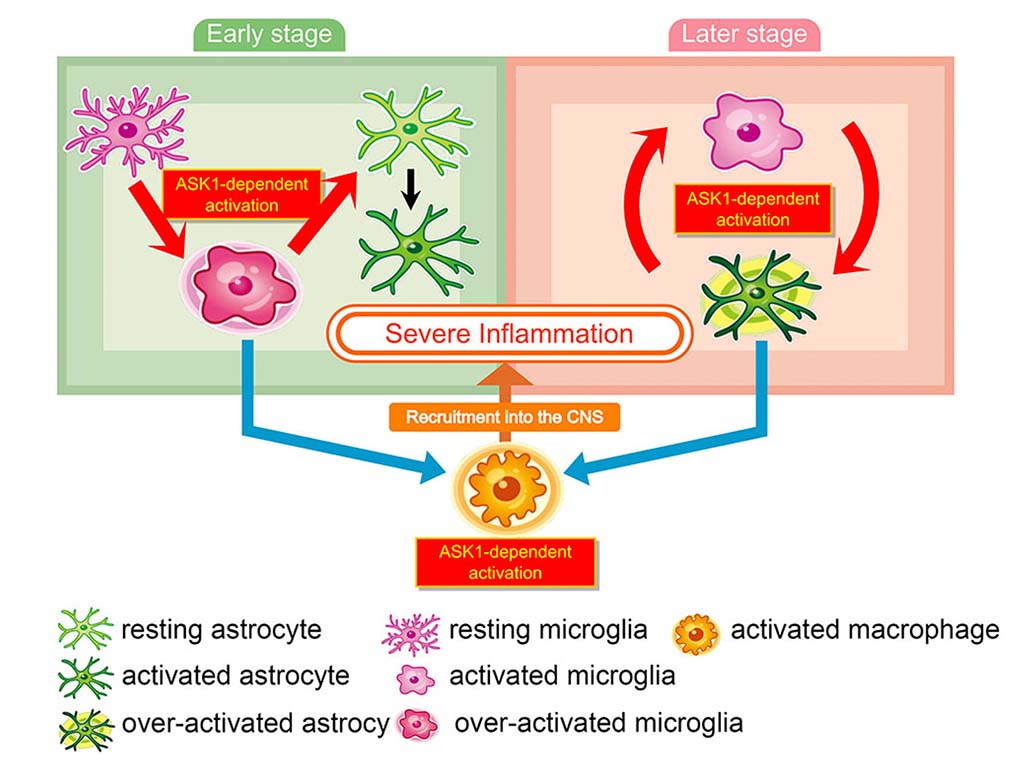
The research group examined cell-type specific roles of ASK1 during experimental autoimmune encephalomyelitis (EAE), an animal model of MS, by generating five conditional knockout mice that lack ASK1 in T-cells, dendritic cells, microglia/macrophages, or astrocytes (Figure 1). They found that neuroinflammation was reduced in both the early and later stages of EAE in microglia/macrophage-specific ASK1 knockout mice, whereas only the later stage neuroinflammation was ameliorated in astrocyte-specific ASK1 knockout mice (Figure 2). ASK1 deficiency in T cells and dendritic cells had no significant effects on EAE severity. Further, ASK1 in microglia/macrophages induces a pro-inflammatory environment, which subsequently activates astrocytes to exacerbate neuroinflammation. On the other hand, activated astrocytes produce key inflammatory mediators, including CCL2, that further activated and recruited microglia/macrophages, in an astrocytic ASK1-dependent manner. Astrocyte-specific analysis revealed CCL2 expression was higher in the later stage compared with the early stage, suggesting a greater proinflammatory role of astrocytes in the later stage. Their findings demonstrate cell type-specific roles of ASK1 and suggest phase-specific ASK1-dependent glial cell interactions in EAE pathophysiology (Figure 3). These finding suggest that glial ASK1 is a promising therapeutic target for reducing neuroinflammation.
This work was supported by JSPS KAKENHI Grants-in-Aid for Scientific Research, the Japan Agency for Medical Research and Development (AMED), the Takeda Science Foundation, the Suzuken Memorial Foundation, the Naito Foundation, and the Uehara Memorial Foundation.