A research team led by Dr. Daisuke Yamane, Moe Matsumoto and Kotomi Shinozaki at the Viral Infection Control Project in Tokyo Metropolitan Institute of Medical Science has discovered an innate immune mechanism that promotes constitutive protection against virus infections. Host cells are known to be protected by multiple layers of innate antiviral defense: virus-induced signaling that is activated upon cellular sensing of specific pattens associated with invading viruses, and constitutive signaling that helps to maintain the basal expression of antiviral factors. However, the regulatory mechanism that controls the constitutive protection has been understudied, because its functional importance has not been recognized until recently. Results published in Nucleic Acids Research show that CSNK2B, a ubiquitously expressed protein, is an important component that supports constitutive antiviral signaling.

The research team had investigated the regulatory mechanisms of cell-intrinsic antiviral immunity and discovered the existence of a constitutive layer of host defense, primarily driven by interferon regulatory factor 1 (IRF1) present in the nucleus, where it drives expression of antiviral genes (Nat Microbiol. 2019 4:1096-1104.). IRF1 transactivates a set of genes that mediate antiviral effector functions against a broad range of viruses, such as hepatitis viruses and flaviviruses. However, it has remained uncharacterized how IRF1 activity is regulated post-translationally by cellular factors.
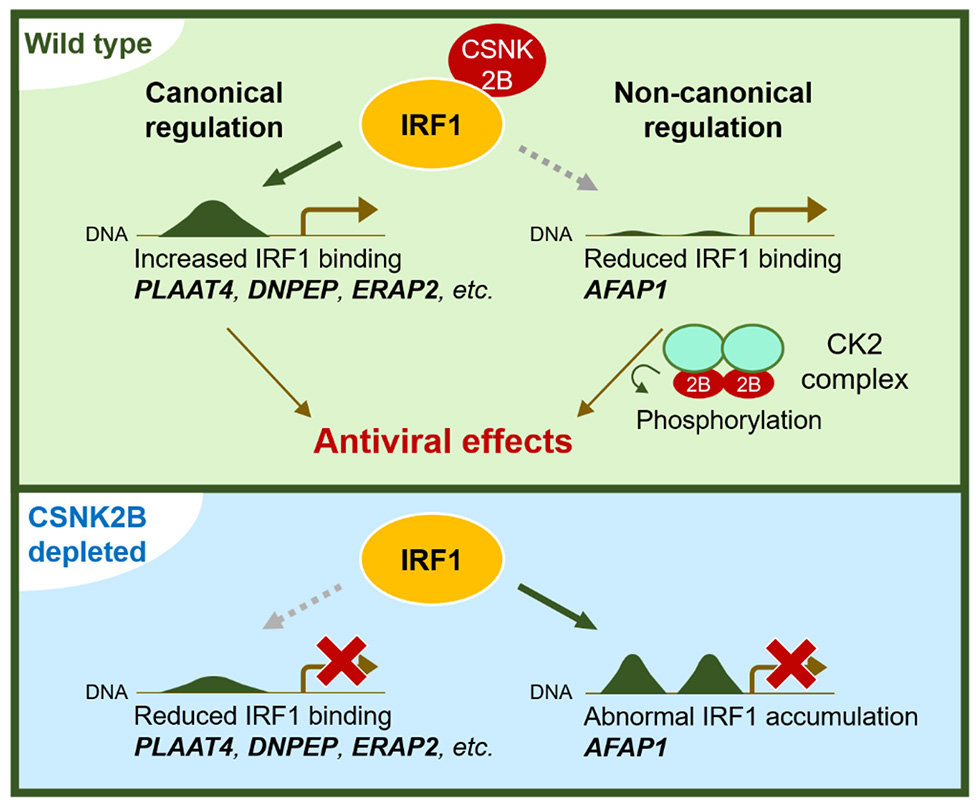
In an attempt to discover host factors affecting IRF1 activity, the research team employed a proteomics approach to isolate proteins interacting with IRF1. This led to identification of CSNK2B, a regulatory subunit of casein kinase 2 (CK2), as an IRF1 cofactor that enhances its transcriptional activity. In the absence of CSNK2B, IRF1’s activity to drive antiviral effector genes was reduced to approximately half of the wild-type levels.
Genome-wide investigation of IRF1 binding loci revealed reduction in the binding of IRF1 to chromatin in CSNK2B-depleted cells, thereby affecting transcription of many known IRF1-regulated antiviral genes, such as PLAAT4 and ERAP2. These results demonstrate an important role of CSNK2B in enhancing the IRF1 binding to its target sites. The only exception to this finding was that silencing expression of CSNK2B induced abnormal accumulation of IRF1 at AFAP1 loci, which paradoxically functions as a brake to down-regulate transcription of AFAP1. In either case, CSNK2B acts as an enhancer for the IRF1-regulated gene transcription.
AFAP1 (actin filament associated protein 1) encodes an actin crosslinking factor that is implicated in oncogenic growth via Src activation. Importantly, CSNK2B, as part of a CK2 complex, was also found to mediate phosphorylation-dependent activation of AFAP1-Src signaling and exert suppressive effects against flaviviruses, including dengue and Zika viruses.
These findings demonstrate a post-translational mechanism of IRF1 regulation and identify new effector genes mediating important cellular functions governed by CSNK2B, CK2, and IRF1. Reports have shown that CK2 is a proviral factor for replication of hepatitis C virus, human immunodeficiency virus, and SARS-CoV-2, etc., but we and others show the activity is associated with antiviral effects against other viruses, such as flaviviruses and influenza virus. Further clarifying the mechanisms by which CSNK2B modulates host antiviral functions would likely provide the basis for developing antiviral and anticancer therapies.