Tauopathy is characterized by the accumulation of filamentous tau in neurons and/or glial cells. In Alzheimer's disease (AD), all tau isoforms expressed in the human adult brain appear as neurofibrillary tangles and undergo various post-translational modifications such as phosphorylation and ubiquitination. On the other hand, in Pick's disease, progressive supranuclear palsy, and corticobasal degeneration (CBD), tau isoforms with three or four repeated sequences in the microtubule-binding domain form pathologies that characterize each disease. Recently, classification of tauopathies based on tau filament core structure and disease-specific post-translational modifications have been demonstrated. We previously reported a cultured cell model of seeded tau aggregation induced by patient-derived tau seeds. However, the structure of tau filaments amplified in the cells and the role of post-translational modifications in templated seeding were not clear.
Recent studies have indicated that templated amplification of tau filaments is a mechanism underlying the progression of tauopathy. In this study, we investigated whether tau filaments amplified in our cellular model exhibit the structural and biochemical properties of the original seeds.
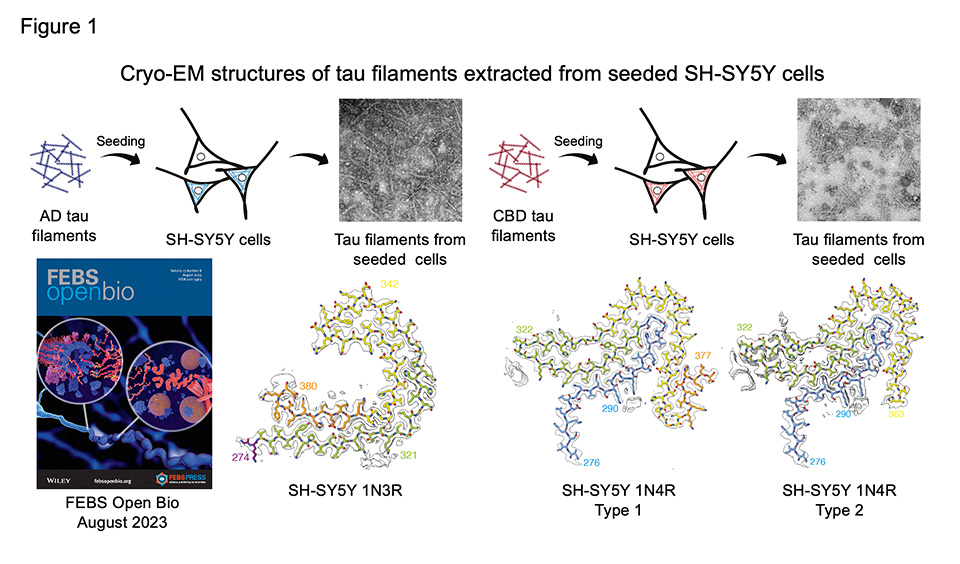
First we introduced tau seeds extracted from AD and CBD brains into human neuroblastoma SH-SY5Y cells expressing HA-tagged human tau. 3 days after introduction, the detergent-insoluble fraction was extracted from the seeded cells, and the structures of SH-SY5Y-cell-derived tau filaments were determined by single particle analysis using cryogenic electron microscopy. Seeded AD and CBD filaments showed a filament core structure(s) resembling each original seeds (Figure 1). However, seeded CBD filaments did not contain the identical non-proteinaceous molecules as CBD seeds, which resulted in structural differences at the N- and C-terminal core ends. In addition, seeded AD and CBD filaments were identified as protofilaments. These results indicated that the introduction of patient-derived seeds did not simply induce tau filament formation in SH-SY5Y cells, but that it occurred in a template-dependent manner. It has been also suggested that some cellular environmental factors and non-proteinaceous molecules are involved in the formation of disease-specific tau filaments. This work was conducted in collaboration with Dr. Sjors H. W. Scheres and Dr. Michel Goedert at MRC Laboratory of Molecular Biology, UK.
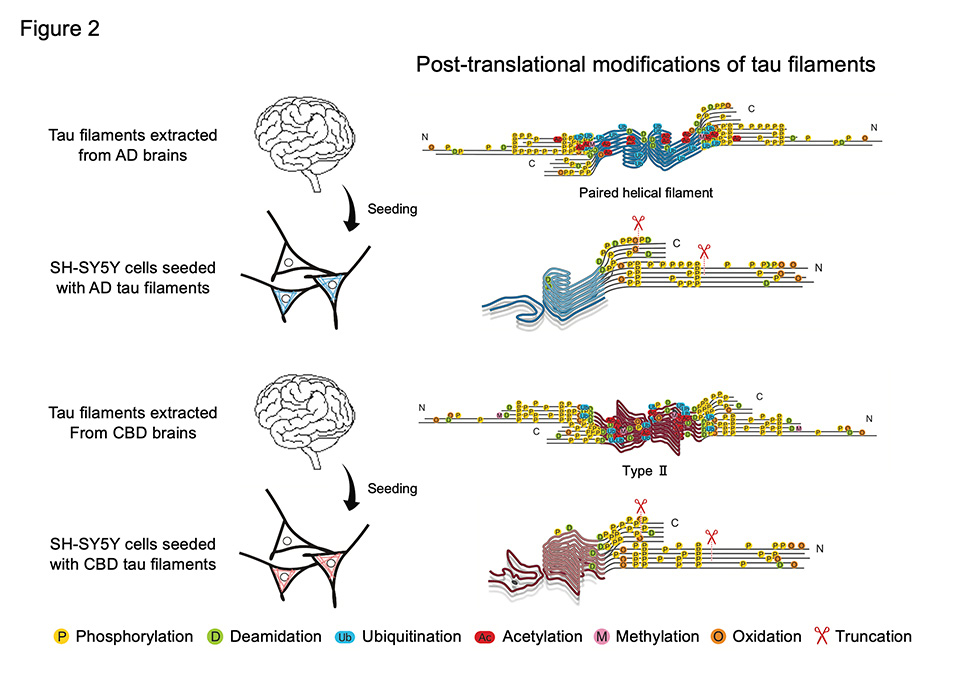
Furthermore, we examined post-translational modifications of SH-SY5Y-cell-derived tau filaments by mass spectrometry and compared them with those of the original seeds. Most of phosphorylation, deamidation, and oxidation detected in the original seeds were reproduced in insoluble tau extracted from seeded SH-SY5Y cells. In addition, it has been suggested that tau post-translational modifications increase from the non-filament core region, called "fuzzy coat," to the filament core region, and that disease-specific ubiquitination and acetylation are prominent in more matured tau filaments. These results indicate that templated amplification of tau filaments is initiated by the presence of template tau and that the profile of tau post-translational modifications changes with the intracellular accumulation of filaments.
The two studies demonstrated that seeded tau aggregation observed in cultured cells occurs based on structural information of seeds and that tau post-translational modifications increase associated with filament formation. This templated tau filament formation may be an essential process in the clinicopathological diversity of tauopathies. The cultured cell model used in this study was also shown to be useful in tauopathy research and in the development of novel therapeutic strategies.
These works were supported by the Japan Agency for Medical Research and Development (AMED), the Japan Science and Technology Agency (JST), the Japan Society for the Promotion of Science (JSJP) Grants-in-Aid for Scientific Research, UKRI MRC and Alzheimer's Research UK.