Stem cell project team in our institute developed an efficient in vitro differentiation induction culture system of human induced pluripotent stem cells into macrophages. This study was published in the journal of the Japanese Society of Immunology.

Human induced pluripotent stem cell (hiPSC)-derived hematopoietic cells, such as red blood cells, white blood cells, and platelets, are promising research materials for cancer immunotherapy, regenerative medicine, and drug discovery. Therefore, we are researching in vitro hematopoietic induction system from hiPSCs. Previously, we demonstrated that the use with CHIR99021, an inhibitor of GSK3β, greatly enhanced hematopoietic differentiation from hiPSCs (Kitajima et al. 2015). Based on the finding, we could recently induced immunophenotypical hematopoietic stem/progenitor cells (HSPCs), namely CD45+CD34+LIN–CD38–CD90+ cells (hereafter abbreviated as hiPS-HSPCs), from CHIR99021-treated hiPSCs by a novel three-dimensional organoid culture system (Kitajima et al. 2023).
However, these hiPS-HSPCs did not exhibit long-term repopulating (LTR) activity, a hallmark of somatic HSPCs, suggesting that there is a missing cue in hiPS-HSPCs, which is critical for retaining LTR activity. Therefore, we compared the gene expression of hiPS-HSPCs with human cord blood-derived HSPCs (hCB-HSPCs) and found that a receptor tyrosine kinase FLT3, which is highly expressed in hCB-HSPCs, is mostly absent in hiPS-HSPCs (Kitajima et al. 2023).
As FLT3 is activated by its ligand, the cytokine FLT3L, we introduced FLT3 into hiPSCs and the resultant hiPSCs (FLT3-hiPSCs) were induced into HPSCs by organoid system in the presence of FLT3L. Unfortunately, hiPS-HSPCs were unable to acquire LTR activity by FLT3/FLT3L activation. Instead, a large amount of myeloid progenitor cells, which are direct progenitors of immune cells such as macrophages and neutrophils, could be obtained by FLT3/FLT3L activation (Kitajima et al. 2023).
Macrophages are innate immune cells that prevent bacterial infections by phagocytosis and remove dead cells for keeping tissue homeostasis. Macrophages also play pivotal roles in stimulating acquired immunity, such as T lymphocytes, by secreting cytokines/chemokines.
As mouse macrophages and their immortalized cell lines are commonly used for their research, it remains unknown whether the findings obtained in mice would be fully relevant to humans or not. If hiPS-derived macrophages can be used for research, it will make a significant contribution to human macrophage research.
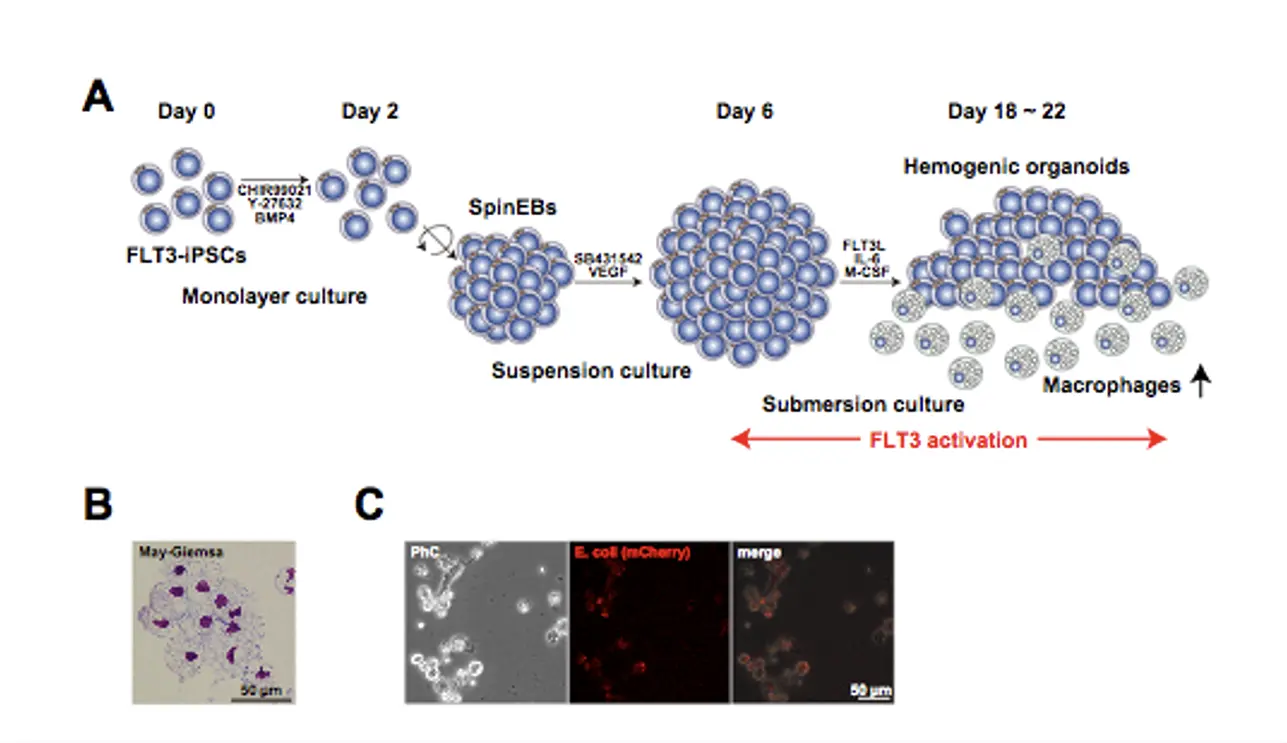
As mentioned above, FLT3/FLT3L activation significantly promotes the induction of myeloid progenitor cells from hiPSCs. Therefore, it is likely that FLT3/FLT3L activation would also be useful for the production of macrophages from hiPSCs. As expected, we found that large numbers of macrophages were produced from hiPSCs by FLT3/FLT3L activation in the macrophage induction culture condition (Fig.1A). Further we demonstrated that these macrophages displayed comparable functions to normal macrophages (Fig.1B & C).
Macrophages could engulf and eliminate tumor cells when artificial receptors (chimeric antigen receptor; CAR), that specifically recognize cancer cells, are introduced. These receptors, CARs, are already used in T lymphocytes to treat leukemia and other tumors (CAR-T therapy). In future, macrophages would be useful for cancer immunotherapies. We believe that hiPS-derived macrophages would contribute to the development of these new therapeutic strategies as research materials.