
It is well established that apolipoprotein B (ApoB) plays a central role in the packaging and export of lipids from liver cells in the form of very low-density lipoproteins (VLDL). This process is crucial for preventing excessive lipid accumulation in the liver. The packaging of neutral lipids into VLDL particles depends on the activity of Microsomal Triglyceride Transfer Protein (MTP), which transfers triglycerides to ApoB to initiate its secretion.
The importance of ApoB and MTP in maintaining liver lipid homeostasis is supported by genome-wide association studies (GWAS), which have identified genetic polymorphisms in the APOB and MTP gene loci as risk factors for metabolic dysfunction-associated steatotic liver disease (MASLD). Interestingly, recent GWAS findings have also pointed to polymorphisms in the APOE gene as a contributor to MASLD, but the precise function of ApoE in regulating fat accumulation in the liver has remained unclear.
In a new study, a research team led by Dr. Daisuke Yamane and Kotomi Shinozaki, a graduate student at Ochanomizu University, along with Professor Ikuyo Ichi from Ochanomizu University, investigated the potential compensatory role of ApoE in hepatic lipid metabolism under conditions where ApoB function is compromised. The researchers, members of Immunity Control Unit of Research Center for Infectious Diseases Medical Sciences, used human hepatoma cells, a model system that actively secretes VLDL, to explore this functional relationship.
They found that inhibition of ApoB secretion, either by pharmacological blockade using an MTP inhibitor (lomitapide) or by genetic knockout, led to a marked increase in the expression and secretion of ApoE into the culture medium. Interestingly, while ApoB secretion is strictly dependent on MTP activity, ApoE secretion occurred independently of MTP, but was found to require active triglyceride (TG) synthesis mediated by the enzymes DGAT1 and DGAT2 (diacylglycerol O-acyltransferases). These findings suggest that newly synthesized TG act as a trigger for ApoE secretion from the Golgi apparatus, allowing hepatocytes to promote TG export in the absence of functional ApoB-mediated pathways.
To further explore the functional impact of ApoE on lipid turnover, the team compared hepatocytes with intact ApoB function to those treated with MTP inhibitor. Strikingly, inhibition of MTP alone did not increase intracellular TG levels, but a significant accumulation of TG was observed when ApoE expression was also suppressed. This indicates that ApoE upregulation may serve as a compensatory mechanism that maintains lipid export when the ApoB-dependent secretion pathway is disrupted.
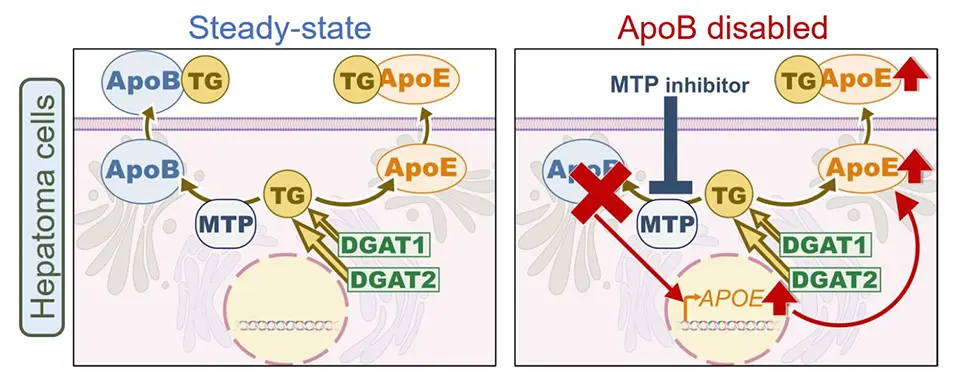
This study offers new insights into the redundant and adaptive mechanisms that regulate TG homeostasis in liver cells. The newly identified compensatory mechanism may also influence the infectivity and composition of hepatitis C and B virus particles, which are known to incorporate ApoE and/or ApoB on their surface during the particle assembly. Furthermore, the ApoE-dependent regulation of TG turnover may have broader implications beyond liver metabolism, potentially contributing to the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease, which is strongly influenced by genetic polymorphisms within the APOE gene locus.