Dr. Chihiro Hisatsune and Dr. Yasuko Ono, (a project leader at the time), of the Calpain Group, published an article entitled “In situ detection of activation of CAPN3, a responsible gene product for LGMDR1, in mouse skeletal myotubes” in the Journal of Biological Chemistry. They succeeded in elucidating the dynamics of calpain 3 (CAPN3) after activation in skeletal myotubes and identified its endogenous substrates. The findings of this research are expected to contribute to understanding of the pathogenesis of LGMDR1, which is caused by CAPN3 deficiency.
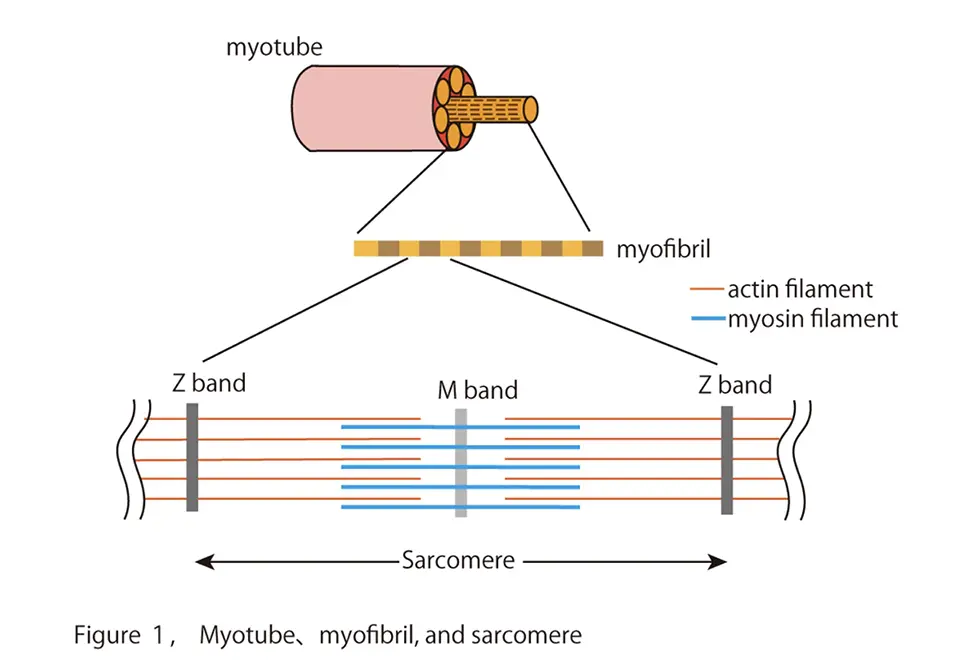
Limb girdle muscular dystrophy R1 (LGMDR1)[1] is an incurable disease characterized by progressive muscle weakness in the proximal muscles of the extremities, affecting about 2 in 100,000 people. Most patients with LGMDR1 develop the disease in adolescence, suffer from gradually weakening muscles, and become wheelchair-bound at their 30s. To date, no curative treatment of LGMDR1 has been established. In the early 1990s, calcium-dependent cysteine protease[2] "calpain [3]3" was identified as the responsible gene product for LGMDR1. Then, numerous researchers have extensively studied biochemical characteristics of CAPN3 to elucidate the pathogenesis of LGMDR1 and revealed several enigmatic features of CAPN3. For example, CAPN3 has an insertion sequence “IS1” in the protease region unlike classical calpain[4]. The insertion sequence renders CAPN3 inactive under resting conditions, and the autolytic removal of IS1 is therefore necessary for CAPN3 to form a catalytic active center, enabling it to hydrolyze other substrates. Calpain 3 also associates with titin[5], a structural protein in the sarcomere[6] of myofibrils[7] in muscle cells (Fig. 1). However, due to its rapid autolytic properties and uncertain substrates in living cells, its activation mechanism and physiological function in skeletal muscles remain unknown.
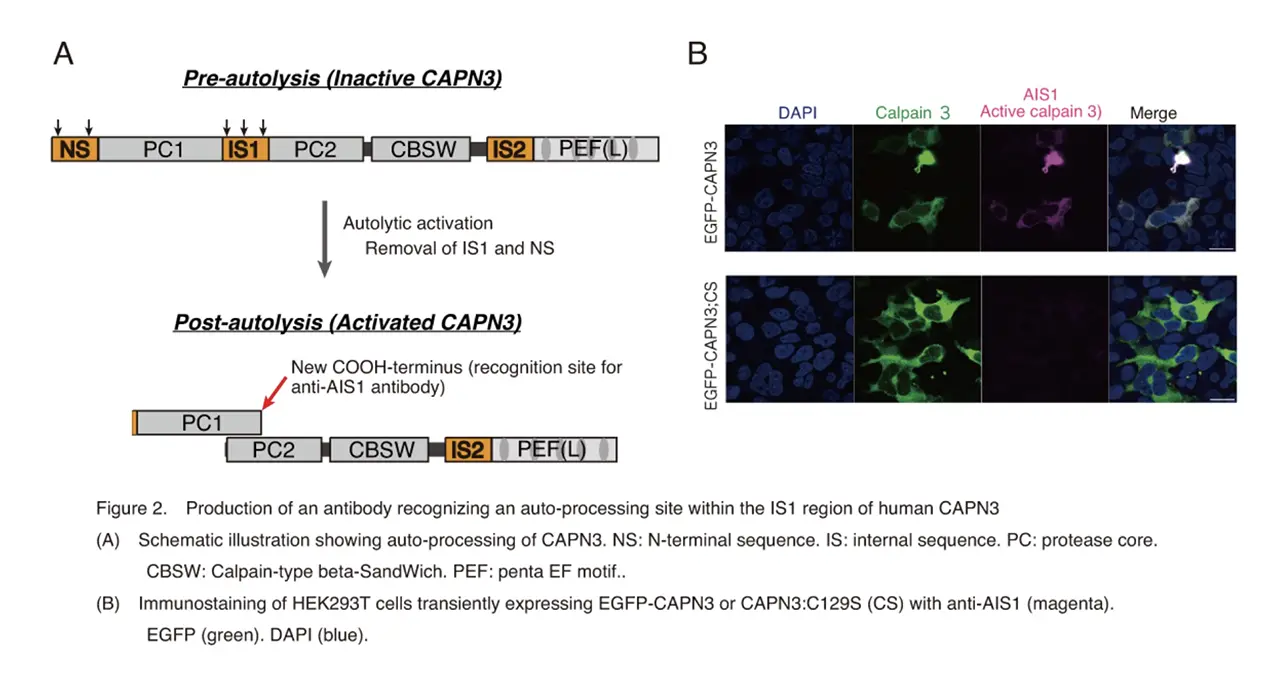
The research group focused on the autolysis of CAPN3 during activation, and challenged to develop an antibody that recognizes the CAPN3’s autolytic processing (Fig. 2A). They succeeded in producing an antibody that differentiate between wild-type CAPN3 and a protease-inactive CAPN3 mutant, and named it Autolytic site within IS1 (AIS1) antibody (Fig. 2B). The antibody visualized activation process of CAPN3, which was exogenously overexpressed in cultured cells, by ouabain, a cardiotonic steroid[8]. They also found that ouabain triggered a small but long-lasting cytoplasmic increase in intracellular calcium [8], which was not sufficient for CAPN1 activation.
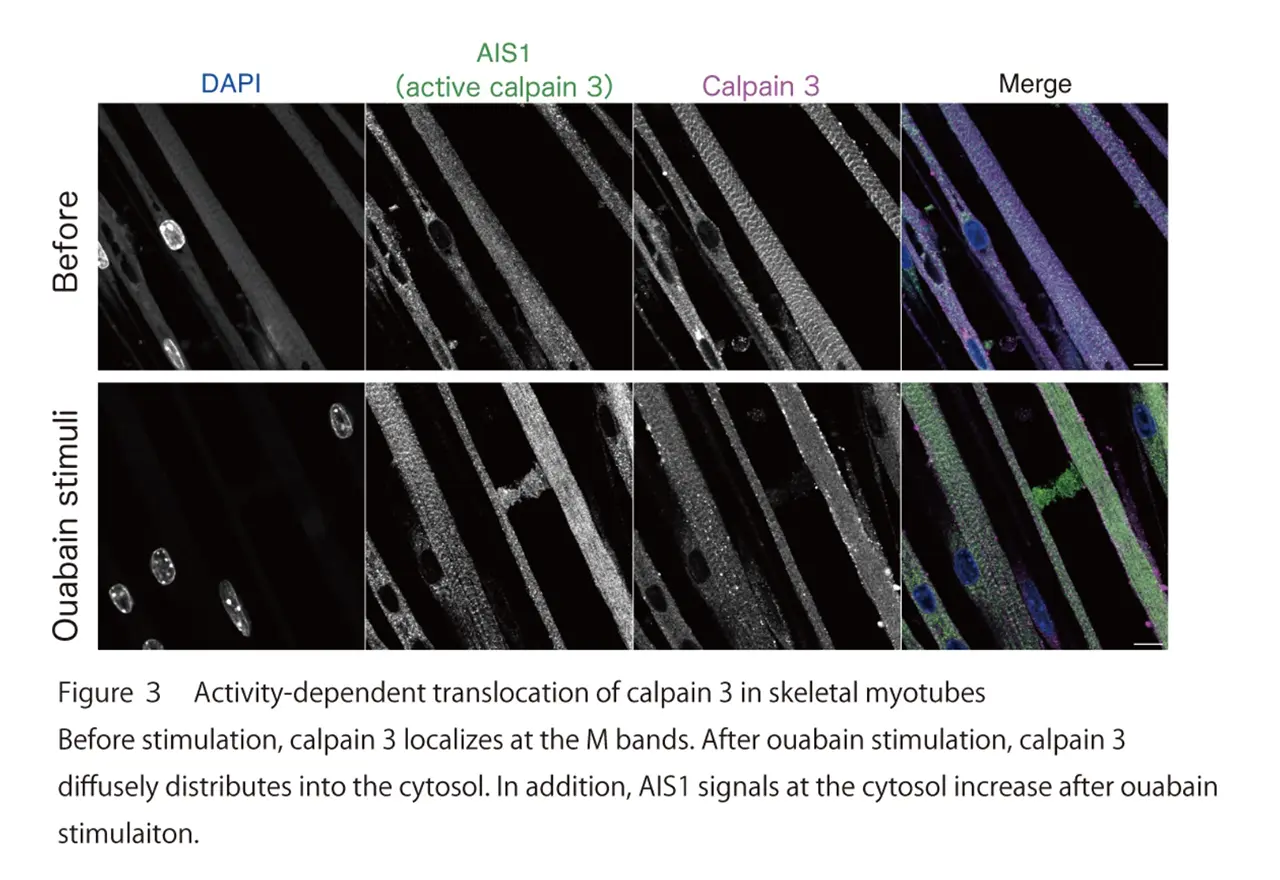
The research group further examined the subcellular localization of endogenous CAPN3 in primary cultured skeletal myotubes. They found that CAPN3 predominantly localizes at the M band of sarcomeres in resting skeletal muscle myotubes. Furthermore, they demonstrated that CAPN3 translocated from the M band into the cytoplasm after ouabain stimulation (Fig. 3). The CAPN3’s translocation was not observed in skeletal myotubes obtained from knock-in mice expressing activity-deficient mutant of CAPN3. Therefore, they concluded that the protease activity of CAPN3 is essential for the translocation of CAPN3.
Finally, the research group examined the endogenous substrates of the activated CAPN3 in skeletal myotubes. They found degradation of spectrin and talin, cytoskeletal proteins that had previously been reported to be digested by CAPN3 in vitro, after ouabain stimulation. These phenomena were not observed in skeletal myotubes from knock-in mice expressing the inactive form of CAPN3, confirming that spectrin and talin are genuine substrates of CAPN3 in skeletal myotubes.
The research group demonstrated that the protease activity of CAPN3 is essential for its translocation from M-bands into the cytoplasm and the subsequent digestion of, at least, two cytoskeletal proteins, spectrin and talin, in intact skeletal myotubes. There are many mutations in CAPN3 with inactive or decreased protease activities in the patients with LGMDR1. Therefore, the failure of degradation of spectrin and talin by CAPN3 may be, at least in part, the underlining pathogenesis of LGMDR1.
It is important future issues to find physiological conditions that cause a slight and long-lasting increase in calcium level in skeletal myotubes and to examine the precise mechanism by which CAPN3 is activated in such a low level of intracellular calcium. Since ouabain application can selectively activate CAPN3, it is a suitable pharmaceutical drug for identifying the CAPN3’ substrates in skeletal myotubes. These future studies will provide more knowledge on the pathogenesis of LGMDR1, which will lead to the development of a new treatment for LGMDR1.
This research was supported by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (C) (Chihiro Hisatsune, Grant Number 22K07014), Grants-in-Aid for Scientific Research (C) (Yasuko Ono, Grant Number 22K06156).