Primary lateral sclerosis (PLS) is a rare motor neuron disease (MND) in which the upper motor neurons are predominantly affected. In contrast, amyotrophic lateral sclerosis (ALS), the most common MND, involves impairment of both upper and lower motor neurons, and most sporadic cases exhibit TDP-43 pathology. PLS is considered to represent part of a continuous spectrum with ALS, but criteria to distinguish PLS from ALS have not yet been fully established. Therefore, the longstanding question has been whether PLS is a subtype of ALS or a distinct disease.
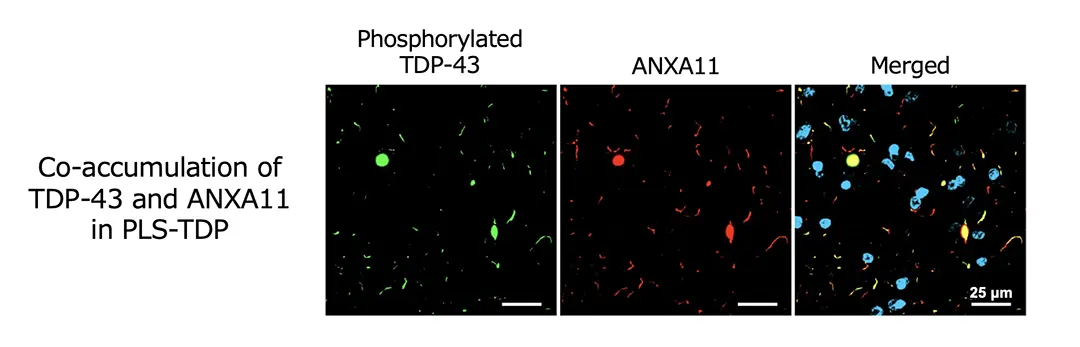
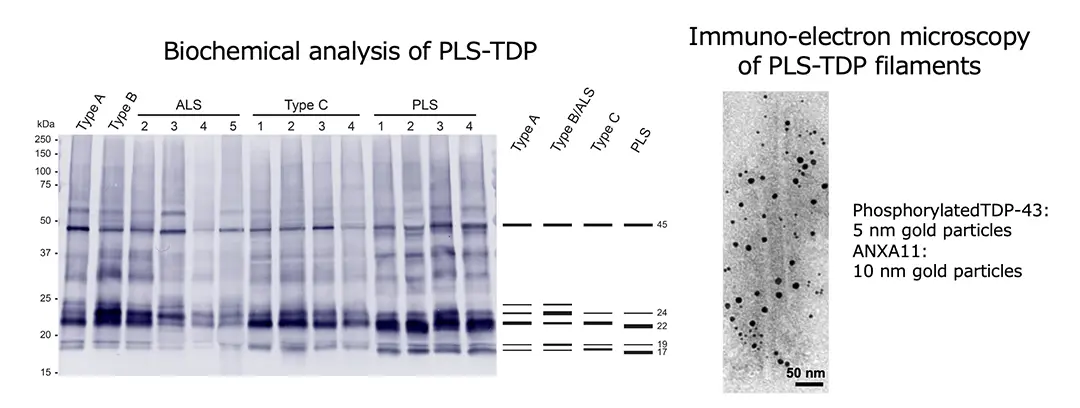
Recently, it has been revealed that annexin A11 (ANXA11) co-aggregates with TDP-43 in the brains of patients with frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP) Type C, and that TDP-43 and ANXA11 form heteromeric amyloid-like filaments. Hence, we re-examined ANXA11 co-aggregation in TDP-43 proteinopathy brains by immunohistochemistry. This led to the finding of ANXA11 accumulation in FTLD/MND clinically presenting with PLS (PLS-TDP). PLS-TDP exhibited phosphorylated TDP-43-positive neuronal cytoplasmic inclusions and dystrophic neurites resembling FTLD-TDP Type A, distinct from ALS, which were ANXA11-positive (Figure 1). Furthermore, biochemical analysis of PLS-TDP brains revealed that PLS-TDP showed a phosphorylated TDP-43 banding pattern distinct from FTLD-TDP Types A, B, and C, indicating biochemical characteristics of PLS-TDP that differ from those of ALS (Figure 2). Finally, immuno-electron microscopy demonstrated that filamentous structures extracted from PLS-TDP brains were co-labelled with phosphorylated TDP-43 and ANXA11 antibodies, suggesting the formation of TDP-ANXA filaments similar to those in FTLD-TDP Type C (Figure 2). Our findings provide evidence that co-aggregation with ANXA11 serves as a neuropathological and biochemical indicator distinguishing PLS from ALS and that ANXA11 is involved in the pathogenesis of PLS-TDP. Further structural studies of PLS-TDP brains and the development of early diagnostic approaches targeting ANXA11 are expected.
This work was supported by the Japan Agency for Medical Research and Development (AMED), the Japan Science and Technology Agency (JST), the Japan Society for the Promotion of Science (JSJP) Grants-in-Aid for Scientific Research, UKRI MRC and Alzheimer's Research UK.