Researchers from the Calpain Group of our institute—Dr. Aya Noguchi (Researcher), Dr. Shoji Hata (Group Leader), Dr. Yasuko Ono (Former Group Leader) —in collaboration with Dr. Hiroshi Shitara (Head of Animal Research Division, Core Technology Support Center, of our institute), Dr. Yasushi Saeki (The Institute of Medical Science, The University of Tokyo), and Dr. Hikaru Tsuchiya (Juntendo University), have discovered that calpain 15 (CAPN15), an intracellular protease, targets ubiquitin-modified proteins and regulates the cell adhesion molecule E-cadherin.
Loss-of-function mutations in CAPN15 have recently been reported to cause a congenital malformation disorder known as oculogastrointestinal neurodevelopmental syndrome (OGIN). Our findings are therefore expected to contribute to a better understanding of the molecular mechanisms underlying this disorder. This work was published online in the Journal of Biological Chemistry.

CAPN15 is a member of the calpain family of intracellular calcium-dependent proteases. Among calpains, CAPN15 is unique in its ability to bind ubiquitin, a small protein that is attached to specific target proteins as a signal for degradation or functional regulation.
In recent years, loss of CAPN15 function has been linked to OGIN, drawing attention to the function of CAPN15 in development and tissue morphogenesis. However, the physiological role of CAPN15 has remained unknown.
In this study, we investigated the function of CAPN15 using epithelial cell culture models. Cells lacking CAPN15 exhibited abnormal morphology characterized by excessive cell crowding.
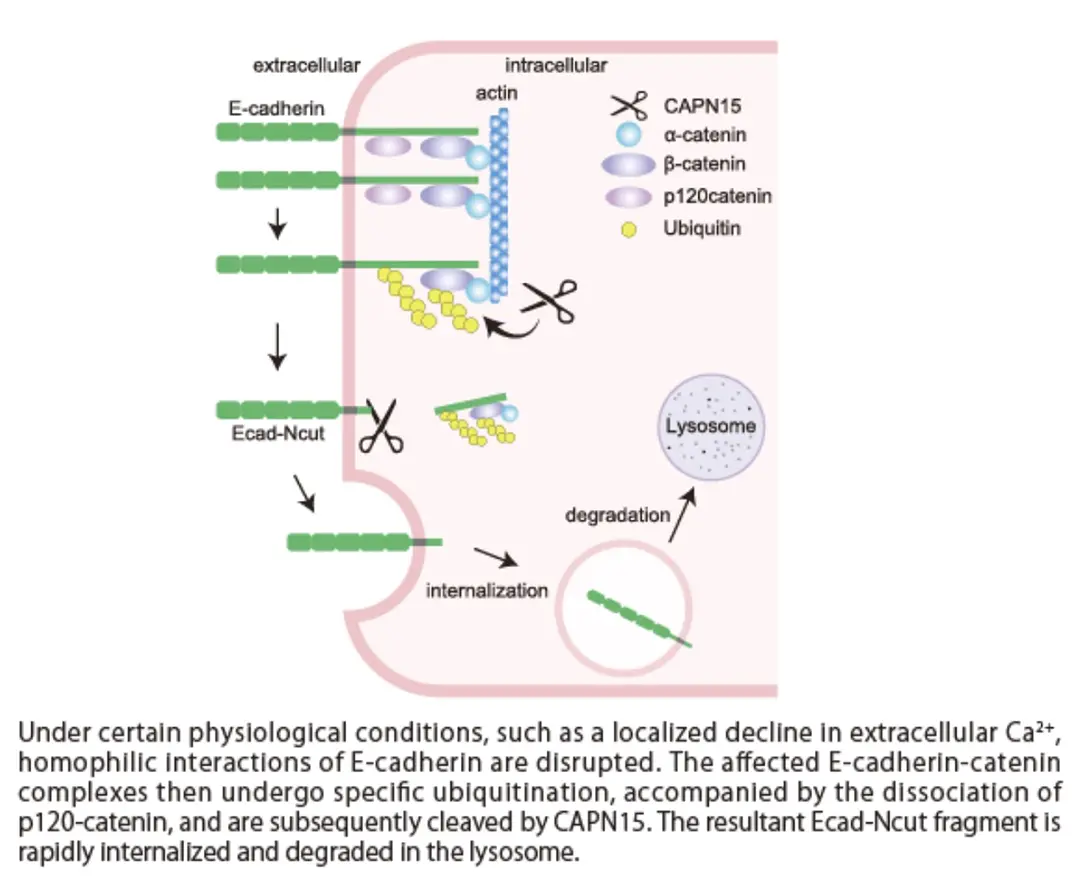
To elucidate the molecular basis of this phenotype, we searched for substrates of CAPN15 and identified E-cadherin, a key cell–cell adhesion molecule localized at the plasma membrane. E-cadherin plays a central role in maintaining epithelial integrity by mediating intercellular adhesion.
Our results demonstrate that, under specific physiological conditions, CAPN15 recognizes ubiquitinated E-cadherin and cleaves it, thereby regulating cell surface E-cadherin levels. When CAPN15 function is disrupted, this regulatory process is dysregulated, leading to excessive accumulation of E-cadherin at the plasma membrane and abnormal cell morphology. Importantly, accumulation of E-cadherin was also observed in epithelial tissues of CAPN15-deficient mice, indicating that this phenotype is recapitulated in vivo.
This study identifies CAPN15 as a highly distinctive protease that uses ubiquitin as a molecular signal to selectively cleave specific target proteins. Proteases that recognize ubiquitin-modified substrates are extremely rare, and our findings provide new insights into previously unrecognized mechanisms of protein regulation.
Proper cell-cell adhesion is essential for maintaining tissue architecture and organ function. As E-cadherin plays a central role in these processes, dysregulation of E-cadherin levels leads to developmental abnormalities and tissue disorganization. Our findings demonstrate that CAPN15 plays a critical role in maintaining epithelial homeostasis by fine-tuning E-cadherin levels.
Because loss-of-function mutations in CAPN15 cause OGIN, this work provides important insights into the pathogenesis of this disease and is expected to facilitate future studies on development and tissue morphogenesis.