RESEARCH
Intracellular quality control is an essential for cells to perform optimally and allows our bodies to maintain a healthy state.
Understanding the molecular mechanisms underlying intracellular quality control is expected to have a significant impact not only on basic biological research but also on a wide range of fields, including medicine and pharmacology.
In this project, we aim to discover novel mechanisms of cellular quality control and to elucidate their operating principles at the molecular level.
Currently, our research focuses on the molecular mechanisms of various forms of “intracellular quality control,” based on the following three themes.
- Discovery of novel organelle stress responses and their corresponding organelle quality control mechanisms
- Identification of protein-specific quality control mechanisms
- Molecular mechanisms of autophagy regulation by novel factors
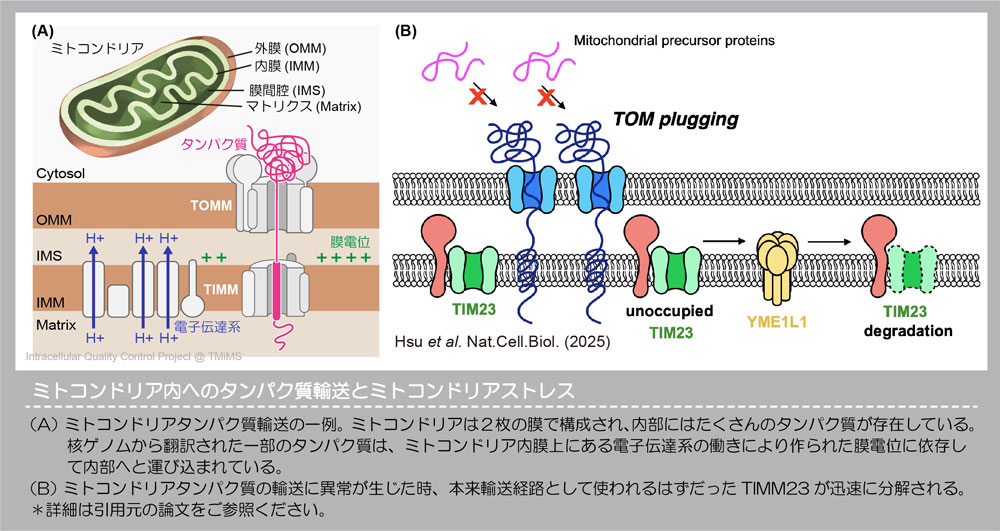
▶ Discovery of new organelle stress and its corresponding organelle quality control mechanism
The various organelles within cells have complex and distinct functions. Therefore, each organelle is likely to experience its own specific types of stress.
For example, mitochondria are composed of two lipid membranes that contain channels (or “gates”) responsible for importing necessary molecules into the organelle. Inside the mitochondria, there are also enzymes (proteases) that degrade proteins.
We recently discovered that clogging of the mitochondrial membrane channels causes stagnation of protein import, leading to stress that affects mitochondrial quality. Furthermore, under these stress conditions, we observed that a specific mitochondrial protein is selectively degraded by a protease.
Our research focuses on these unique types of organelle stress and the corresponding quality control systems that respond to them.
▶ New quality control mechanism specific to a specific functional protein
Proteins function properly within cells only when they adopt the correct three-dimensional structure.
Because many proteins cannot fold into their native conformations on their own, they require assistance from folding intermediaries such as molecular chaperones.
In addition, some proteins function by dynamically changing their structure in response to different ligands.
To maintain the quality of such proteins, a continuous support system—or a “mechanism to prevent structural errors”—is essential. However, this aspect has received little attention until recently. We have identified a novel chaperone-like factor that assists in the folding of proteins undergoing constant structural changes, and we are currently analyzing its molecular function.
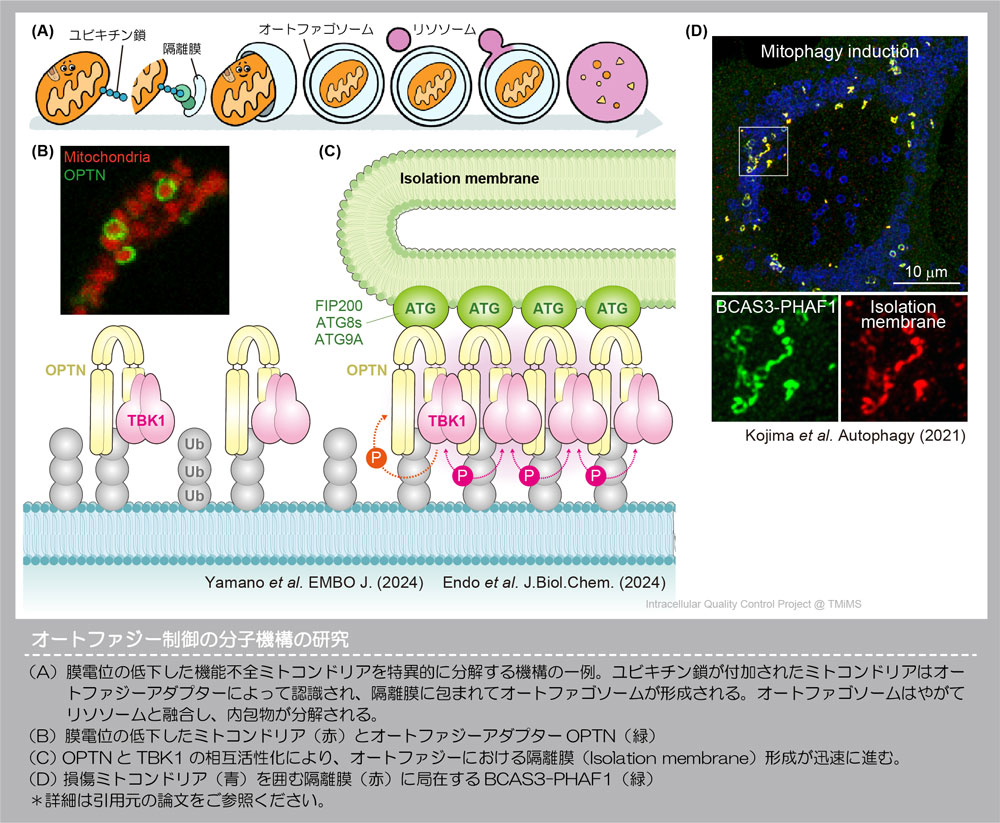
▶ Molecular mechanism of autophagy control by new factors
Autophagy is an intracellular degradation system highly conserved in many species.
During autophagy, dageradation targets are enclosed within a lipid membranes, then degraded upon fusion with the lysosome. Many different proteins are involved in this process.
Recently, we identified a novel proteins in mammalian cells that accumulates on the lipid membranes formed during autophagy. Studies using cultured cells have clarified the mechanisms by which these proteins are localized to lipid membranes and promotes autophagy. We are continuing to investigate its functions, including which molecular activities are critical for autophagy, which remain unclear.
Since one of the newly identified proteins has been reported as a causative gene for neurological diseases in humans, we are investigating the relationship between autophagy failure and disease onset using model organisms. Our goal is to elucidate the molecular mechanisms by which breakdowns in intracellular quality control contribute to disease development.