Research
RESEARCH PROJECT
Glycobiology in the Cytosol and Drug Discovery Based on Glycoprotein-Specific Ubiquitin Ligases
Proteins are glycosylated in the endoplasmic reticulum (ER) or Golgi apparatus. These membrane-bound organelles transport glycoproteins to the extracellular matrix or to other organelles. Because of this transport system, glycoproteins were not thought to be present in the cytosol. However, recent studies have found that glycoproteins transiently appear in the cytosol after bacterial infection, organelle damage, or glycoprotein misfolding.
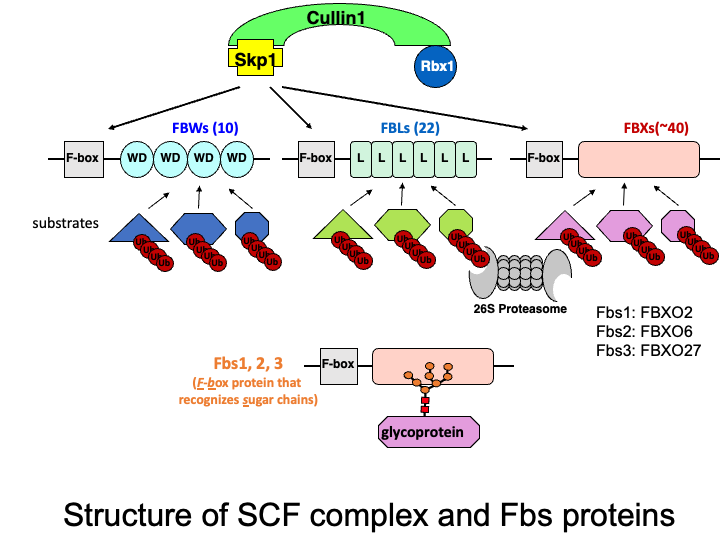
The Skp1-Cullin1-F-box protein containing complex (or SCF complex) is an E3 ubiquitin ligase complex that ubiquitinates proteins to direct them toward proteasomal degradation. F-box proteins are substrate-recognition subunits of the complex, and we previously identified three cytosolic F-box proteins (F-box protein recognizing sugar chains: Fbs1, Fbs2, and Fbs3) that recognize N-linked glycans. Currently we are studying the function of these F-box proteins and identifying target glycoproteins in the cytosol.
☆ Glycoprotein-specific ubiquitin ligases
The ubiquitin-proteasome system is responsible for selective proteolysis in eukaryotes. Ubiquitination is catalyzed by three enzymes-E1, E2, and E3, with the E3 enzyme providing target selectivity. The human genome encodes more than 600 E3 enzymes, and the SCF complex consists of three invariant components (Skp1, Cullin1, and Rbx1), and a variable F-box protein that functions as a substrate recognition protein. Among the 70 F-box proteins in humans, we have been characterizing three F-box proteins that recognize glycans.
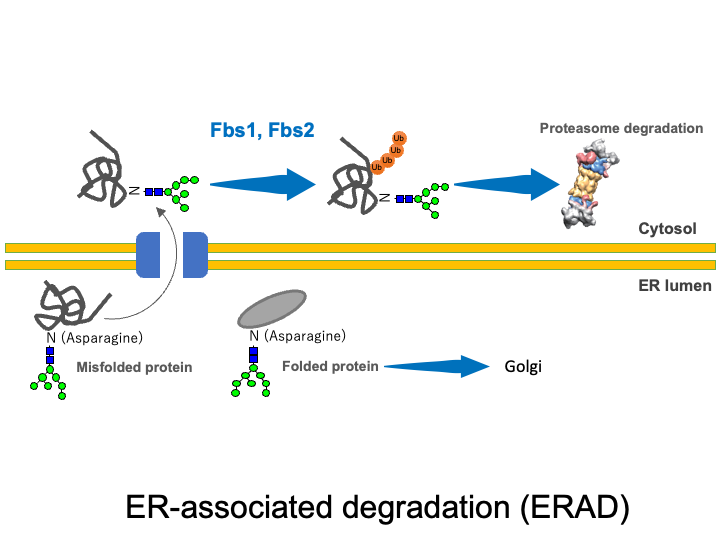
☆ ER-associated degradation (ERAD) pathway
Extracellular proteins such as secretory proteins and membrane proteins are translated in the rough endoplasmic reticulum (ER) and then enter the lumen of the ER, where sugar chains are attached. In the ER, protein folding is carried out with the help of ER chaperones. Proteins that were not folded correctly as well as excess protein subunits are returned from the ER to the cytosol (retrograde transport) where they are degraded by the UPS. This quality control mechanism is known as the ERAD system. SCF complexes containing Fbs1 and Fbs2 are thought to participate in ERAD by recognizing the innermost N-glycans in glycoproteins (the GlcNAc-GlcNAc structure commonly present at the base of N-glycans) that are exposed in denatured proteins or misfolded proteins.
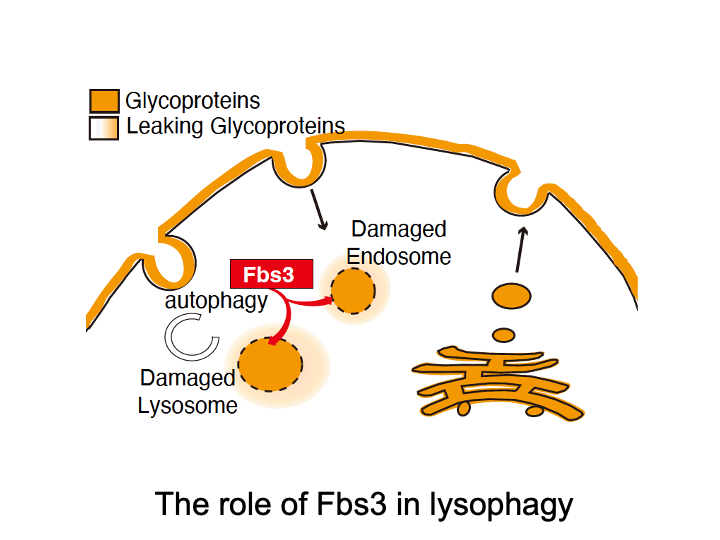
☆ Lysophagy
Fbs3 differs from other F-box proteins because it is myristoylated and localizes to intracellular organelle membranes. When the lysosomal membrane is damaged by bacterial infection or by microparticles taken up from outside the cell, a glycoprotein called LAMP2 becomes exposed to the cytoplasm. SCF complexes containing Fbs3 quickly accumulate around LAMP2 and ubiquitinate many proteins on the damaged lysosome. This induces autophagy and degradation of the damaged lysosome, a process called lysophagy.
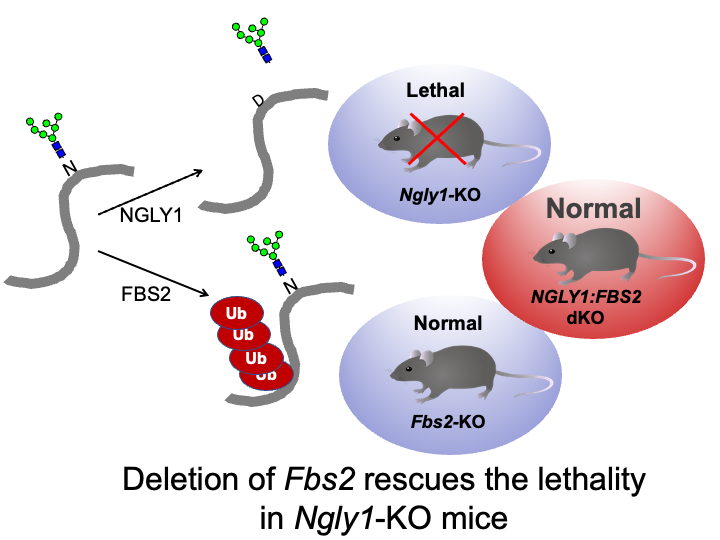
☆ NGLY1 deficiency
Besides glycoprotein-specific ubiquitin ligases, glycosidases that remove sugar chains from proteins are also found in the cytoplasm. N-glycanase (NGLY1) deglycosylates various substrates before they are degraded by the proteasome in the ERAD pathway. Recently, a serious systemic disease called NGLY1 deficiency, an ultra-rare disease with mutations in NGLY1 has been found via exome analysis. Dr. Tadashi Suzuki at RIKEN, who studies NGLY1, reported that Ngly1-KO mice (B6 strain) were embryonic lethal. By chance, we found that mice that are knocked out for both Ngly1 and Fbs2 are born and grow normally. In addition, cell death is observed when Fbs2 is expressed in Ngly1-KO cells, suggesting that Fbs2 is deleterious in NGLY1-KO cells and animals. Based on these findings, we believe that development of inhibitors of Fbs2 may be a promising strategy for treating NGLY1 deficiency and we have currently started screening for these types of drugs with Dr. Suzuki. We are also working to elucidate how Fbs2 causes cytotoxicity.
☆ Ubiquitination mediated by sugar
Dysfunction of the proteasome causes critical damage to cells and organisms, but a compensatory mechanism involving the transcription factor Nrf1 restores proteasome activity. We have discovered that an abnormal ubiquitination of Nrf1 occurs in NGLY1 deficiency. First, this ubiquitination occurs through oxyester bonds on serine or threonine residues near N-glycans, as well as N-GlcNAc residues generated from deglycosylated by ENGASE. Second, SCFFBS2 cooperates with another E3 ligase ARIH1. ARIH1 is known to bind to neddylated Cullin-RING E3 ligases (CRLs) and mediate the addition of the first ubiquitin on substrate, but in this case SCFFBS2 captures N-glycans of Nrf1, and ARIH1 ubiquitinates them. Third, the ubiquitin chains are complex, branched, and elongated through K6, K11, K33, and K48. As a result, the atypical ubiquitin chains inactivate Nrf1 by preventing its nuclear transport. We are currently investigating whether such atypical ubiquitination also occurs in other molecules.
Intracellular Dynamics of the Proteasome
The proteasome is a huge proteolytic enzyme complex assembled of 33 subunits, which was discovered by Dr. Keiji Tanaka, the supervisor of our lab. Dr. Yasushi Saeki (currently a professor at the Institute of Medical Science, The University of Tokyo) analyzed intracellular dynamics of the proteasome by attaching a fluorescent protein to a proteasome subunit. Currently, Dr. Endo in this lab has been analyzing dynamic changes in the proteasome upon various stresses, and unraveling exciting phenomena.
Ubiquitin-mediated organelle stress responses
Ubiquitin chains are known as degradation signals, but they also have various other functions. We have discovered that when organelles are damaged, ubiquitin chains that accumulate on organelle membranes serves a signal for immune response. This stress response is observed not only in endosomes, which we initially discovered, but also in other organelles such as mitochondria and lysosomes, suggesting potential roles in maintaining cellular homeostasis and intercellular signaling.

