
Tuberous sclerosis complex (TSC) is an intractable disease characterized by benign tumors (hamartomas) in the brain, blood vessels, and kidneys. The neuropsychiatric symptoms of TSC include refractory epilepsy, autism spectrum disorders and intellectual disabilities. The genes responsible for TSC are Tsc1 and Tsc2. Their gene products, the Tsc1 and Tsc2 proteins, form a complex that inactivates the small G protein Rheb. Therefore, it has been suggested that the resulting increase in levels of active Rheb (due to mutations in Tsc2) activates downstream signaling cascade proteins, such as the mTORC1 kinase complex, and promotes subsequent neurological symptoms in TSC patients. Everolimus, an mTORC1 inhibitor drug, is effective in treating some tumors and intractable epilepsy and has been approved in Japan. In contrast, treatments for intellectual disabilities and autism have not yet been established.
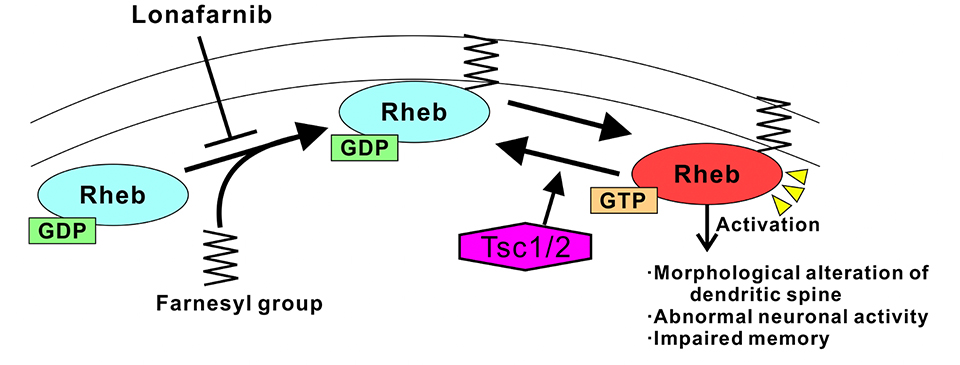
Our research group previously found that synaptic dysfunction in the TSC mouse model was ameliorated by suppressing the activation of the small G protein, Rheb, which regulates mTORC1, rather than by inhibiting mTORC1 activity with everolimus (Sci Rep. 2014; Nature Commun. 2015). Therefore, in the current research, they investigated whether abnormalities in the TSC mouse model could be improved by administering drugs that suppress the function of Rheb (instead of mTORC1). Because Rheb undergoes farnesylation during activation, they used lonafarnib, which suppresses farnesylation of Rheb, as a Rheb inhibitor and examined whether the cellular and behavioral abnormalities in the TSC mice were improved.
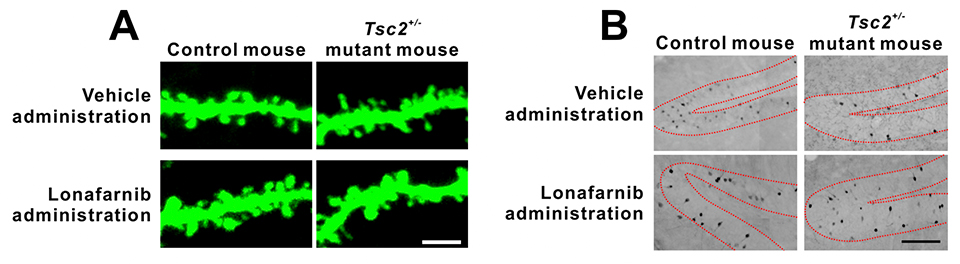
In this research, Tsc2+/- mice were used to model TSC. The cultured neurons of these mice had longer and thinner dendritic spines than those of control mice, but lonafarnib treatment reduced these morphological abnormalities (Fig. 1A). In addition, lonafarnib also recovered the number of spine synapses, which was decreased in the TSC model mice. Similar results were observed in the neurons in the TSC mice brains. These results indicate that lonafarnib reached the brain and influenced the mechanism regulating nerve cell morphology.
As mentioned above, intellectual disabilities are one of the symptoms of TSC. Learning defects are also observed in TSC animal models and are considered to model intellectual disability. Our researchers next investigated whether lonafarnib administration improved memory impairments in Tsc2+/- mice. Learning was recovered when lonafarnib was administered prior to memorization, whereas no recovery was found when lonafarnib was administered subsequent to memorization (but prior to memory recall). In addition, they visualized neural activity during memory recall. The number of activated neurons during recall was decreased in Tsc2+/- mice, but lonafarnib administration prior to memorization restored this number (Fig. 1B). Taken together, these results suggest that the administration of lonafarnib recovered neural morphological abnormalities, restored memorization and reduced memory deficits in Tsc2+/- mice.
Furthermore, our researchers created Tsc2+/- mice with a neuron-specific Rheb deletion. These mice showed normal dendritic spine morphology and no memory impairments. Active Rheb was likely reduced in these mice compared with Tsc2+/- mice, thereby reducing these abnormalities. In conclusion, reducing the levels of active Rheb is an important step in normalizing memory in the TSC mouse model.
Due to the wide range of symptoms of TSC, everolimus has had limited efficacy as a therapeutic drug. The current research indicates that inhibiting Rheb activation via lonafarnib improves neuronal morphological defects, abnormal function, and memory impairments in the TSC mouse model. Moreover, these results suggest that the increase in the levels of active Rheb induces abnormalities in TSC mice (Fig. 2). Because impaired memory in model animals is considered to reflect intellectual disabilities in TSC patients, lonafarnib is a drug candidate for patient treatment. Furthermore, the level of Rheb1 in the brain is increased by epileptic seizures; thus, lonafarnib may also have applications for treating intractable epilepsy as well as TSC and its associated intellectual disabilities in the future.
This work was supported by a Grant-in-Aid for Scientific Research (Kanato Yamagata), the Japan Agency for Medical Research and Development (Kanato Yamagata), the SENSHIN Medical Research Foundation, The Japan Epilepsy Research Foundation (Kanato Yamagata), and the Tokumori Yasumoto Memorial Trust for Research on Tuberous Sclerosis Complex and Related Rare Neurological Diseases.