
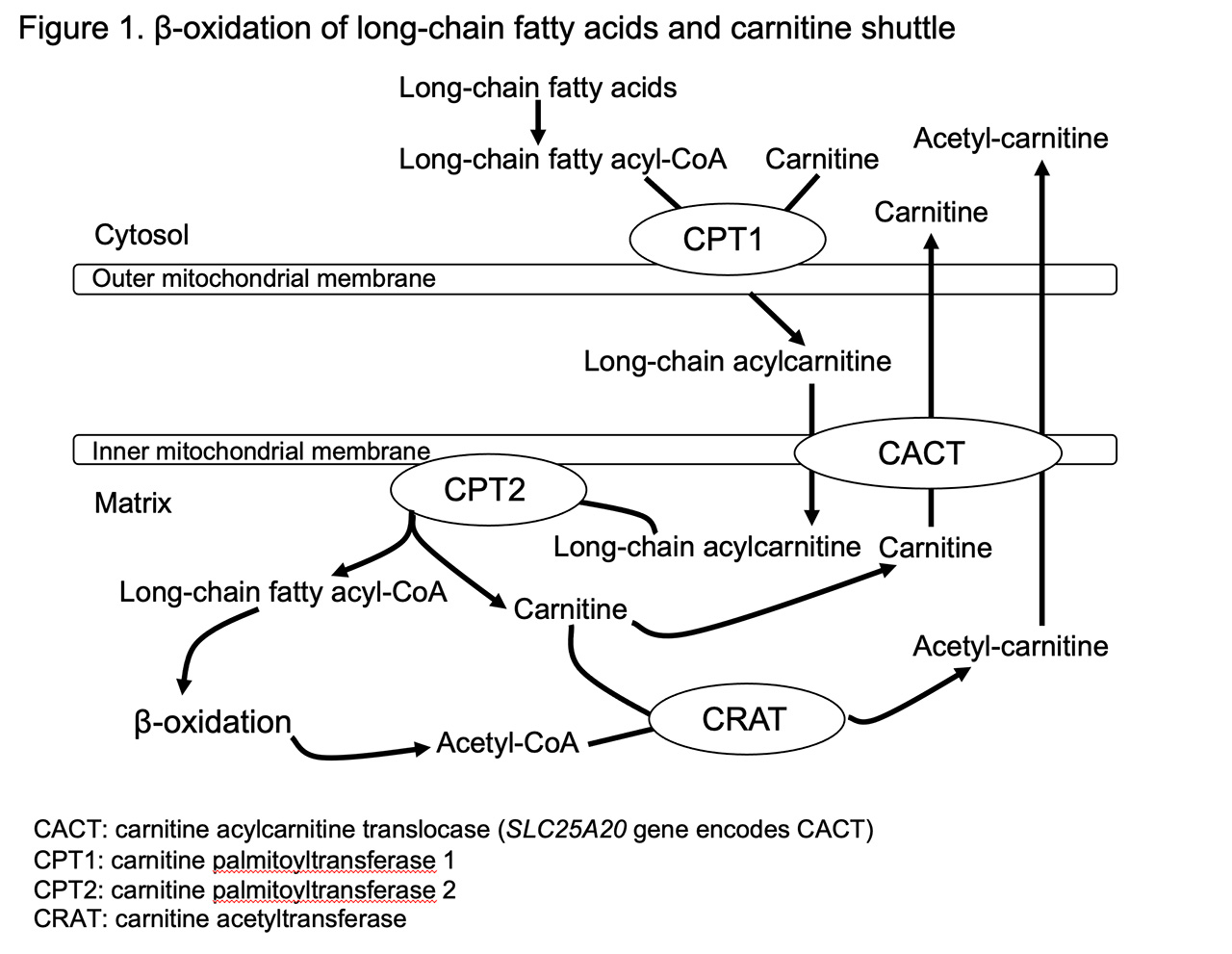
Narcolepsy type 1 (NT1) is characterized by excessive daytime sleepiness, cataplexy, and pathological manifestations of rapid eye movement (REM) sleep, which includes hypnagogic hallucinations, sleep paralysis, or sleep onset REM sleep. We found that a single nucleotide polymorphism (SNP rs5770917) located in carnitine palmitoyltransferase 1B (CPT1B) gene was significantly associated with not only NT1 but also other hypersomnia through a genome-wide association study. The disease risk allele of rs5770917 was significantly correlated with lower expression levels of CPT1B. CPT1B is a rate-limiting enzyme in β-oxidation of long-chain fatty acid. Long-chain acyl-CoAs are converted to long-chain acylcarnitines by CPT1B, which allows their transport into the mitochondria, where they are metabolized through β-oxidation (Figure 1). The transport process for long-chain fatty acids is called the carnitine shuttle. We hypothesized that the production of long-chain acylcarnitines and CPT1 activity are suppressed in NT1 and other hypersomnia. In this study, we determined levels of 25 major individual acylcarnitines in the fraction of red blood cells to clarify the details of altered fatty acid metabolism in NT1 and other hypersomnia. We also analyzed expression profiles obtained by RNA sequencing (RNA-seq) in NT1 patients and healthy controls to assess whether expression levels of genes involved in the carnitine shuttle and β-oxidation are associated with NT1.
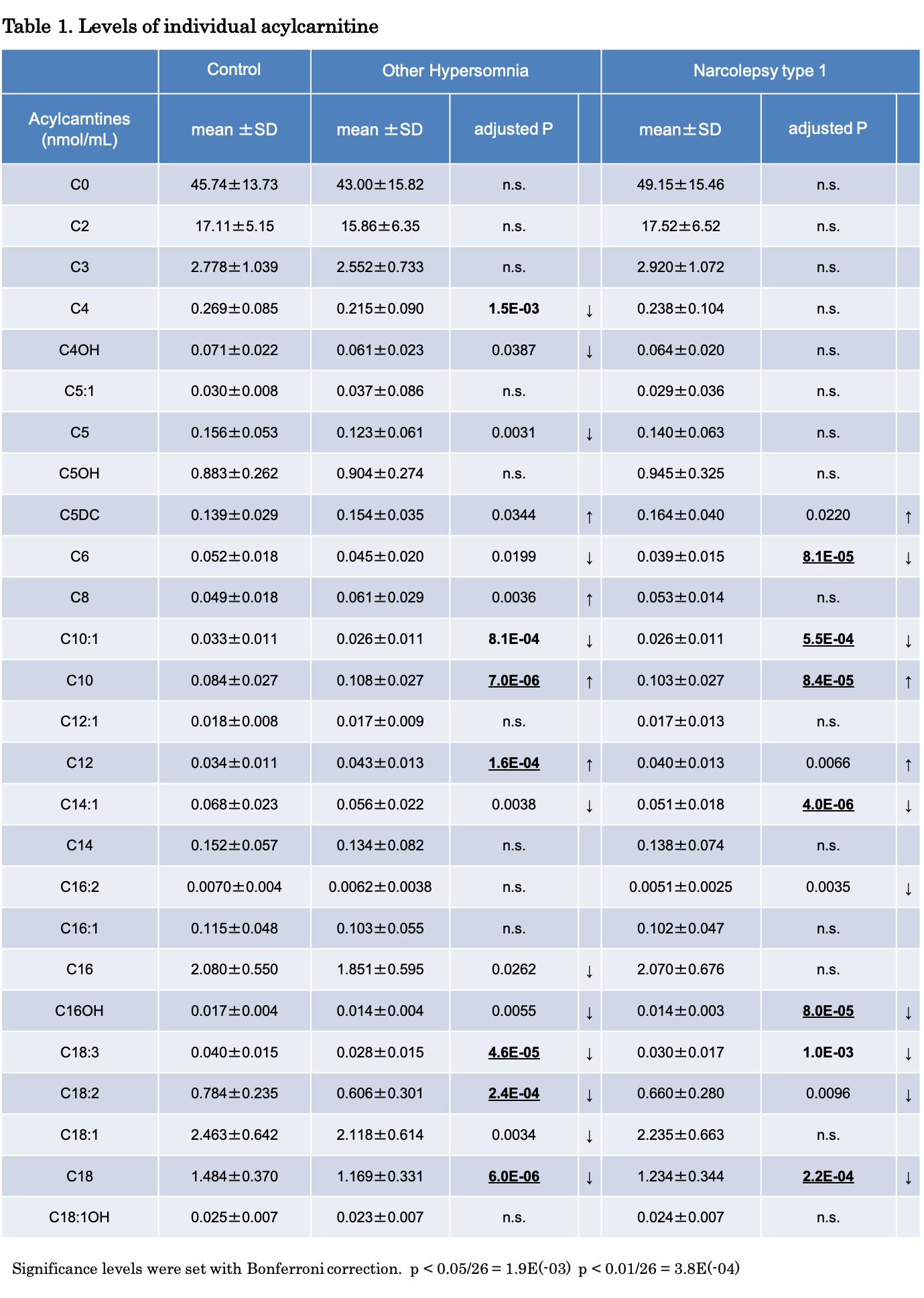
The levels of 25 major individual acylcarnitines in 57 NT1 patients, 51 other hypersomnia patients and 61 healthy controls were determined by electrospray ionization tandem mass spectroscopy. Age, gender, and BMI (body mass index) might affect levels of acylcarnitines. Therefore, we performed a regression analysis using age, gender, and BMI as covariates to compare levels of each acylcarnitine between patient and control groups. As a result, several long-chain acylcarnitines with a carbon chain length of 16 or 18 were found to be low in the narcolepsy group as well as in the other hypersomnia group, compared to the control group (Table 1).
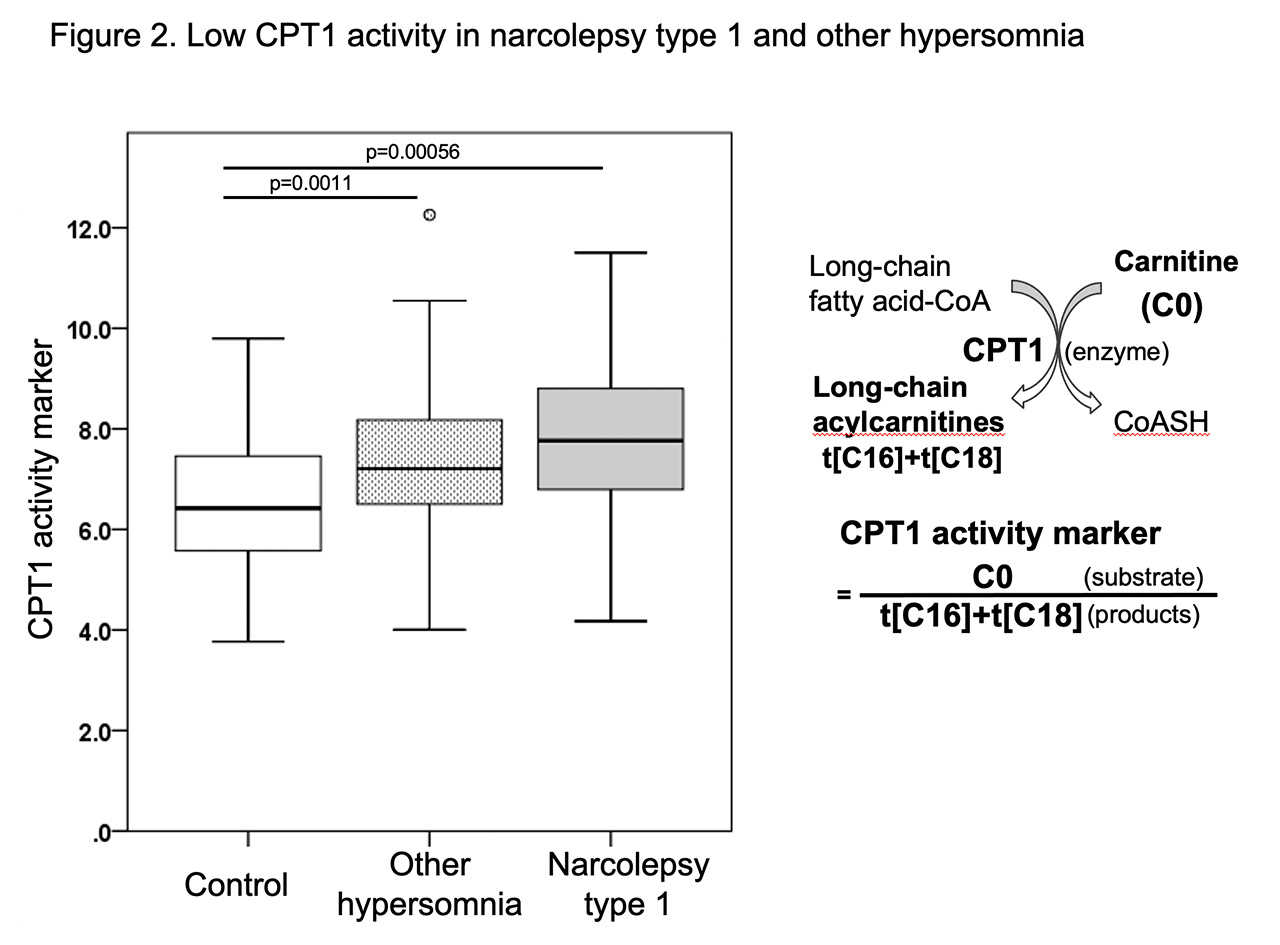
The ratio of C0/(t[C16]+t[C18]) was calculated as a CPT1 activity marker, where C0 (free carnitine) was a substrate of CPT1 and t[C16]+t[C18] was the sum of nine long-chain acylcarnitines that were products of CPT1 (Figure 2). Decreases in CPT1 activity cause a decrease in the CPT1 product (t[C16]+t[C18]), denominator, resulting in the increase of this ratio. Lower CPT1 activity was found to be an independent risk factor for both NT1 and other hypersomnia.
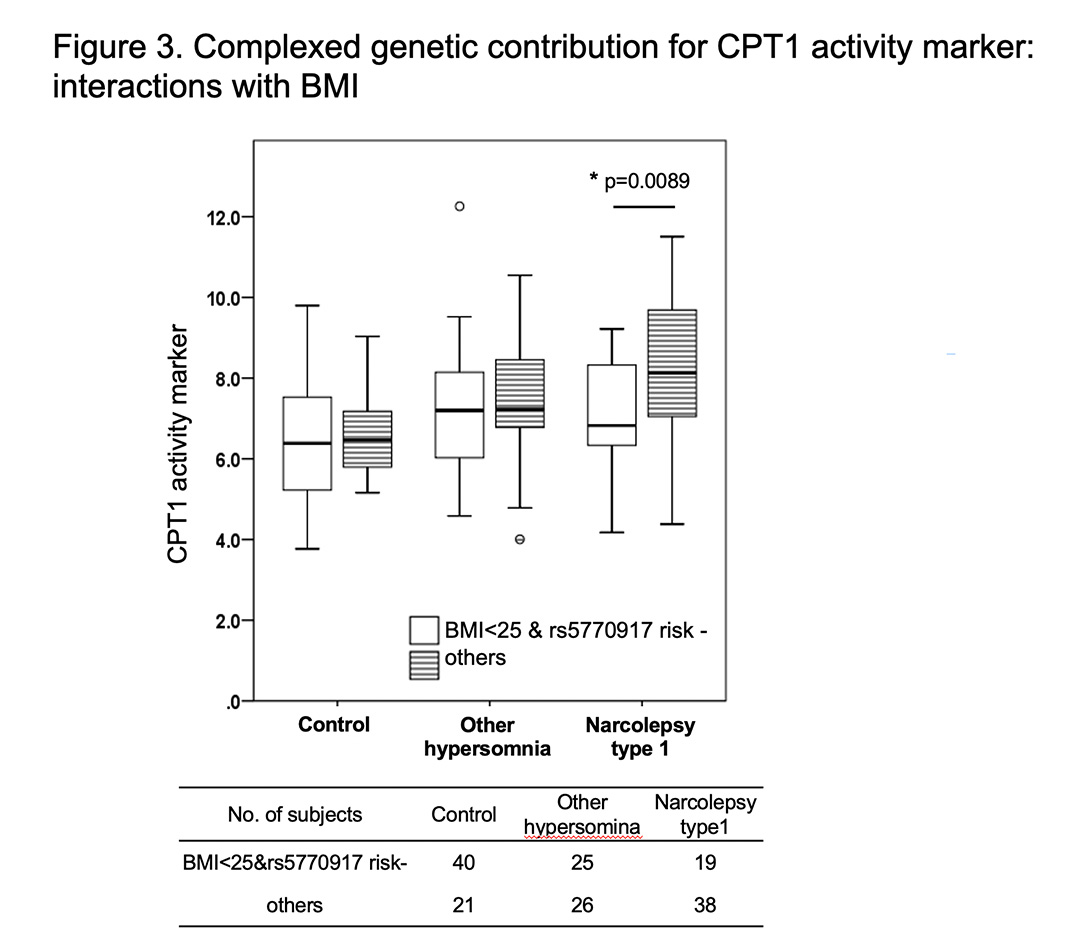
We investigated whether NT 1 and other hypersomnia associated SNP (rs5770917) in CPT1B gene affect CPT1 activity, because rs5770917 is significantly associated with lower expression levels of CPT1B gene. No significant association was observed between rs5770917 and CPT1 activity in general. However, a significant interaction between BMI (<25, ≥25) and SNP rs5770917 (risk+, risk-) was detected and non-obese NT1 patients (BMI <25) without the risk allele had higher CPT1 activity (Figure 3).
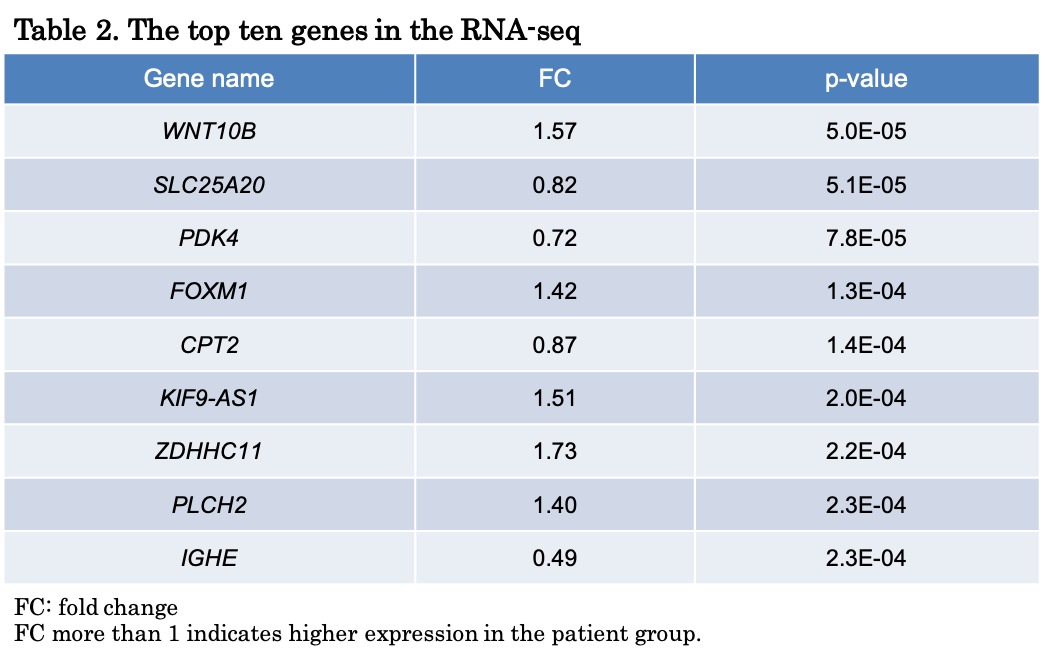
A transcriptome analysis using RNA-seq was conducted in the whole blood samples from 42 NT1 patients and 42 healthy controls to identify differentially expressed genes in NT1. Participants were medication free and fasting upon awakening at the time of blood collection. SLC25A20 gene encoding carnitine-acylcarnitine translocase (CACT) and carnitine palmitoyltransferase 2 (CPT2) gene involved in carnitine shuttle had the second and fifth lowest p-values, respectively, and expression levels of the two genes were lower in the patient group (Table 2). Pathway analyses based on the RNA-seq data showed that the carnitine shuttle was enriched in differentially expressed genes of NT1. CPT1B gene was not included in the differentially expressed genes. The risk allele of rs5770917, a genetic factor for NT1, suppresses CPT1B expression. No SNPs significantly correlated with SLC25A20 and CPT2 expression levels were found. These results suggested that CPT1B expression levels are downregulated due to the genetic factor that is associated with NT1, while SLC25A20 and CPT2 expression levels in NT1 patients are downregulated by factors other than genetic factors.
Our acylcarnitine analysis and RNA-seq based transcriptome analysis provide evidences that abnormal fatty acid metabolism is critically involved in the pathophysiology of hypersomnia. The interaction of rs5770917 in CPT1B and BMI suggested the complexed regulation of CPT1 activity. Controlled studies with larger sample sizes are necessary to replicate our results especially about clinical relevance. CPT1 activity could be used as a biomarker to screen for certain sleep disorders, including NT1 and other hypersomnia.