Drs. Tadayuki Shimada, Kuniko Kohyama, and Kanato Yamagata (Child brain project) and their colleague Dr. Tomoyuki Yoshida (Toyama University) published an article entitled “Neuritin controls axonal branching in serotonin neurons: A possible mediator involved in the regulation of depressive and anxiety behaviors via FGF signaling.” in The Journal of Neuroscience.

Stress is linked to depression and anxiety disorders. Chronic stress treatment leads to decreases in axonal branching, dendritic branching, and the length and number of dendritic spines in the hippocampus, prefrontal cortex, and some amygdala nuclei, which underlies the development of depressive mood in model animals. However, the precise molecular mechanisms by which chronic stress alters neural moephologies are still elusive. Serotonin has been thought to be a main player in the mechanism of depression. A correlation between decreased serotonin and depression was suggested, and the effectiveness of selective serotonin reuptake inhibitors (SSRIs) as antidepressants supports the idea that depression is the result of abnormalities in serotonin signaling in the brain. However, how low-level serotonin induces depressive mood is still unknown.
Our research group previously found that Neuritin contributed the development of refractory epilepsy through the axonal branching of granule cells in dentate gyrus of hippocampus (J. Neurosci. 2016). In this research, we elucidated the function of neuritin on the axon of serotonin neurons and depression and anxiety behaviors. In addition, because it has been also indicated that Neuritin was involved in FGF signaling, we examined the relationship between FGF signaling and depression and anxiety behaviors.
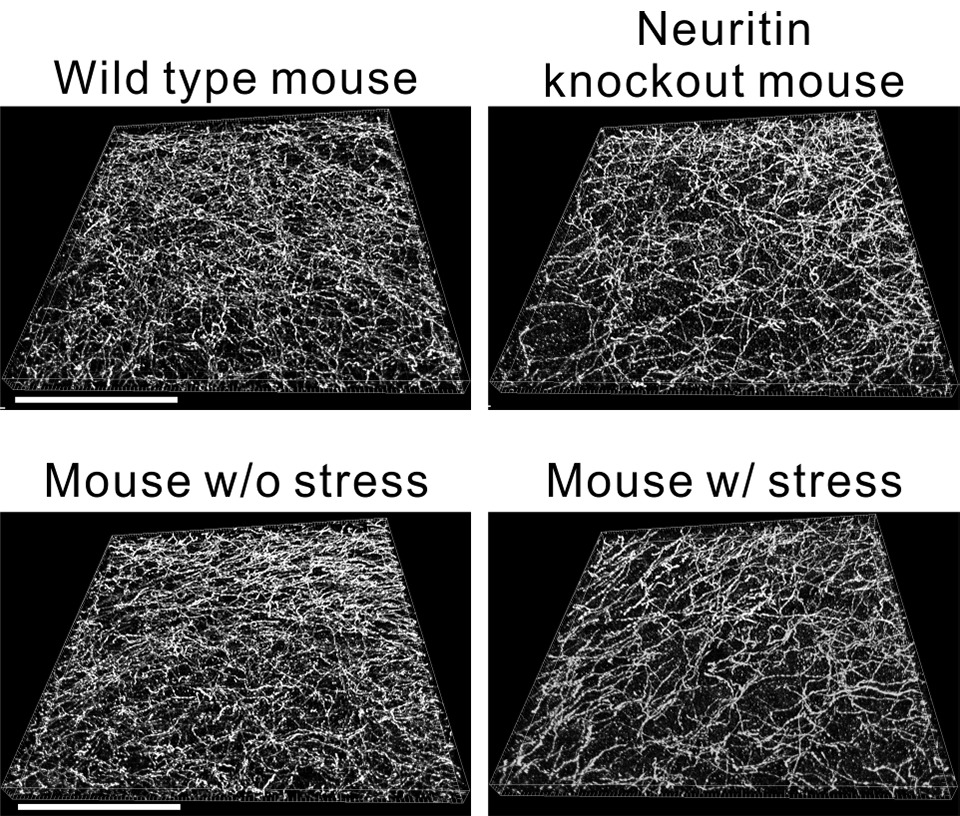
Application of purified Neuritin protein to the culture medium of serotonin neuron promoted the branching of serotonin neuron axons. Serotonin neurons taken from neuritin -/- (knockout) mice exhibited poor axonal branch formation. These results suggest that dose of Neuritin is involved in the formation of axonal branches of serotonin neurons. We next investigated the effect of neuritin on the branching of serotonin neurons in vivo. We visualized the axon of serotonin neurons and observed them in medial prefrontal cortex (mPFC), hippocampus, and basolateral region of amygdala (BLA) where serotonin neurons project their axon. Quantification of the number of axonal branch and density of axon of serotonin neurons revealed that neuritin-KO mice exhibited fewer dense axons and a smaller number of axonal branches in the mPFC and BLA (Figure 1 Top). The number of serotonin neurons was not reduced in KO mice. This result indicates that the loss of neuritin impaired the axonal branch formation of serotonin neurons in the mPFC and amygdala.
Because a decrease in the density of serotonin neuron axons in the amygdala is one of the hallmarks of depression and anxiety, we investigated the influence of neuritin knockout on behavioral models of depression and anxiety in mice. The neuritin KO mice showed depressive and anxiety behaviors, suggesting that the loss of neuritin may be involved in the low axonal branching of serotonin neurons in the mPFC and BLA, which is related to depressive and anxiety behaviors in mice.
Next, we examined the relationship between neuritin and depressive and anxiety behaviors caused by stress, and we found that lower protein expression of Neuritin was detected in the mPFCs, hippocampi, and amygdalae of stress group mice than in those of control mice. Stress conditioned mice also exhibited depressive behaviors and poor axonal branching of serotonin neurons in the mPFC and the BLA (Figure 1 Bottom). Taken together, these results support the hypothesis that chronic stress decreases Neuritin protein expression to impair the axonal branching of serotonin neurons in the mPFC and BLA and that decreased neuritin-mediated axonal branch impairment is involved in the development of depressive and anxiety behaviors in mice. To test directly whether neuritin is involved in stress-mediated axonal morphology regulation and the development of depression and anxiety, we investigated the influence of overexpression of neuritin in the amygdala on mouse behavioral models of depression and anxiety. Overexpression of neuritin blocked stress-mediated axonal branching impairment and development of depressive behaviors. Therefore, it is indicated that Neuritin is involved in the regulation of the axonal branching of serotonin neurons in the amygdala and that the stress-mediated reduction in the expression of Neuritin could be one of the factors controlling the axonal morphology of serotonin neurons in the development of depression and anxiety in mice.
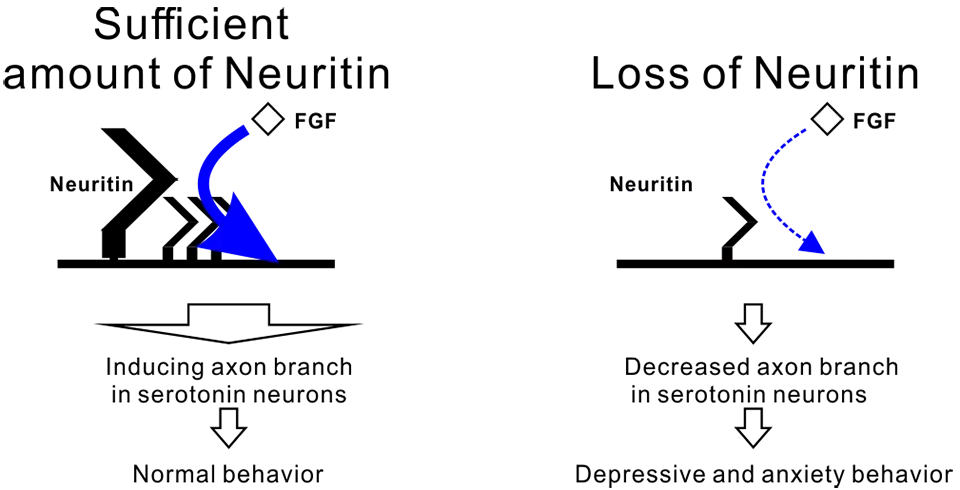
At the last, we focused on the molecular mechanisms by which neuritin regulates the axonal branching of serotonin neurons. We previously reported that Neuritin regulate FGF signaling in granule cells. Therefore, we investigated the involvement of Neuritin and FGF signaling in axonal branching in serotonin neurons. Then, we found that inhibiting FGF signaling reduced axonal branching under Neuritin treatment conditions. In addition, FGF-2 had an effect on the axonal branch formation of cultured serotonin neurons, and neuritin is necessary to promote FGF-2-dependent axonal branching. Next, we evaluated the importance of FGF signaling in the development of depressive and anxiety behaviors. We orally administered FGF signal inhibitor to mice, followed by behavioral tests and preparation of brain sections from these mice. Inhibitor-treated mice presented poor axonal branching and axonal density in the mPFC and amygdala, and inhibitor administration promoted depressive and anxiety behaviors in mice. Our study suggested that a chronic stress-mediated reduction in neuritin expression decreases axonal branch formation in serotonin neurons through regulating FGF signal in the brain and that this neuritin-dependent axonal branching may be involved in stress-mediated depressive and anxiety behaviors in mice (Figure 2).
Originally, low levels of serotonin in the brain were thought to contribute to the development of depressive disorders, and treatment to increase serotonin levels was thought to improve symptoms of depressive disorders; however, recent reports suggested that simply increasing serotonin levels using SSRI treatment does not directly lead to improvements in depressive symptoms. Therefore, the serotonin level in the brain may not directly regulate symptoms of depressive disorders, and researchers have focused on determining the role of increased serotonin levels in regulating neuronal morphology and synaptic transmission. Our study may suggest the possibility to present other therapeutic target to treat depressive and anxiety disorders.
This work was supported by a Grant-in-Aid for Scientific Research.