
- HOME
- Calpain Project
Calpain Project
Molecular mechanism of calpain functions in health
Research Summary
Proteins are chains of amino acids, and their functions change when they are cut or partially cut. Calpains are proteolytic enzymes that perform such cuts or limited proteolytic processing in cooperation with calcium. Humans have 15 calpain species. Defects of these species cause various deficiencies, such as muscular dystrophy, stomach ulcers, and embryonic lethality.
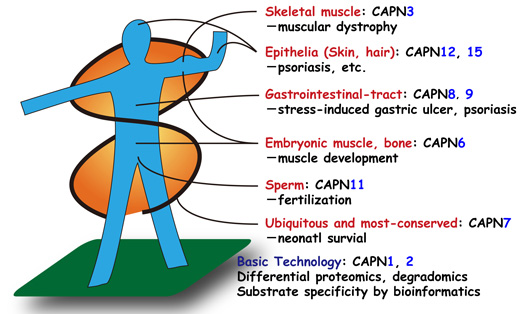
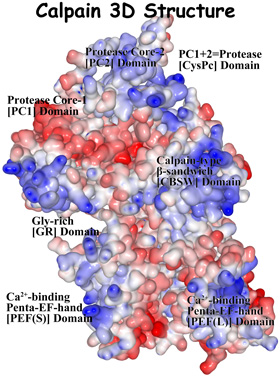
In this project, we aim to understand the biology of calpains, and translate this knowledge into improvements in health.
Selected Publications
- Shinkai-Ouchi F, et al. (2020) “Calpain-2 participates in the process of calpain-1 inactivation.” Biosci. Rep., 40: BSR20200552
- Hata S, et al. (2020) “A muscle-specific calpain, CAPN3, forms a homotrimer.” Biochim. Biophys. Acta, Proteins Proteomics, 7: 140411.
- Hata S, et al. (2016) “A gastrointestinal calpain complex, G-calpain, is a heterodimer of Capn8 and Capn9 calpain isoforms, which play catalytic and regulatory roles, respectively.” J. Biol. Chem., 291: 27313-27322.
- Ono Y, et al. (2016) “Calpain research for drug discovery: challenges and potential.” Nature Reviews: Drug Discovery,15: 854-876.
- Shinkai-Ouchi F, et al. (2016) “Predictions of cleavability of calpain proteolysis by quantitative structure-activity relationship analysis using newly determined cleavage sites and catalytic efficiencies of an oligopeptide array.” Mol. Cell. Proteomics, 15: 1262-1280.
- Ono Y, et al. (2014) “The N- and C-terminal autolytic fragments of CAPN3/p94/calpain-3 restore proteolytic activity by intermolecular complementation.” Proc. Natl. Acad. Sci. USA, 111: E5527-5536.

