
- HOME
- Laboratory of Neural Development
Laboratory of Neural Development

Laboratory Head
Haruo Okado
Brief summary of research
Brain Development and Maintenance
Various factors control differentiation of neural stem cells and survival of the resulting neurons, and aberrancy in these processes are associated with intellectual disability, age-related brain disorders, and brain tumors.We aim to elucidate the mechanisms of development and maintenance of brain functions, ultimately to develop methods for the prevention and treatment of intractable cranial nerve diseases.
“We are studying the effects of various genetic and environmental factors on the molecular mechanisms of brain development and maintenance, with the ultimate goal of developing new treatments for mental diseases.”
Our major projects include
- Understanding how the transcriptional repressor, RP58, regulates brain development and maintenance.
- Altering the nutritional environmental factors to manipulate brain development and functions.
- Understanding the roles of environmental factors in development and aging of brain functions.
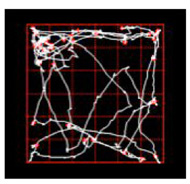
Locomotion, anxiety, memory, and sociality of mice are evaluated using the tracking system. Neuronal activity can be analyzed in vivo system.
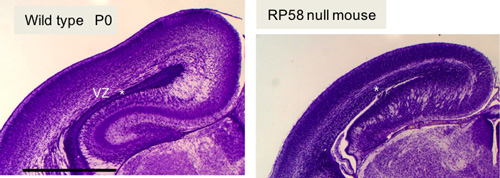
RP58 is required for development of cerebral cortex. The cell-cycle exit of progenitor cells, neuronal radial migration and maturation of cortical neurons are impaired in RP58-deficient mice.
Members
Laboratory Head Haruo Okado
- Shinobu Hirai
- Tomoko Tanaka
Selected Publications
- Hirai S, Hotta K, and Okado H. (2018) “Developmental Roles and Evolutionary Significance of AMPA-Type Glutamate Receptors.” Bioessays. 2018 2018 Sep;40(9):e1800028.
- Hirai S, Hotta K, Kubo Y, Nishino A, Okabe S, Okamura Y, and Okado H. (2017) “AMPA glutamate receptors are required for sensory-organ formation and morphogenesis in the basal chordate.” Proc. Natl. Acad. Sci. USA. 114: 3939-3944.
- Nakajima K, Hirai S, Morio T, and Okado H. (2015) “Benzodiazepines induce sequelae in immature mice with inflammation-induced status epilepticus.” Epilepsy & Behavior 52: 180-186.
- Ohtaka-Maruyama C, Hirai S, Miwa A, Heng JI, Shitara H, Ishii R, Taya C, Kawano H, Kasai M, Nakajima K, and Okado H. (2013) “RP58 regulates the multipolar-bipolar transition of newborn neurons in the developing cerebral cortex.” Cell Rep. 3: 458-471.
- Hirai S, Miwa A, Ohtaka-Maruyama C, Kasai M, Okabe S, Hata Y, and Okado H. (2012) “RP58 controls neuron and astrocyte differentiation by downregulating the expression of Id1-4 genes in the developing cortex.” EMBO J. 31: 1190-1202.
- Ohtaka-Maruyama C, Hirai S, Miwa A, Takahashi A, and Okado H. (2012) “The 5’-flanking region of the RP58 coding sequence shows prominent promoter activity in multipolar cells in the sub- ventricular zone during corticogenesis.” Neuroscience 201: 67-84.
- Okado H, Ohtaka-Maruyama C, Sugitani Y, Fukuda Y, Ishida R, Hirai S, Miwa A, Takahashi A, Aoki K, Mochida K, Suzuki O, Honda T, Nakajima K, Ogawa M, Terashima T, Matsuda J, Kawano H, and Kasai M. (2009) “Transcriptional repressor RP58 is crucial for cell-division patterning and neuronal survival in the developing cortex.” Dev. Biol. 331: 140-151.