
- HOME
- Protein Metabolism Project
Protein Metabolism Project
Mechanisms and Therapeutic Strategies of Protein Metabolism Diseases
Research Summary
The ubiquitin-proteasome system (UPS) is a crucial protein degradation system that affects almost all cellular functions in eukaryotic cells. Since protein homeostasis is essential to human health, malfunctions of the UPS cause various diseases including cancers, inflammation, and neurodegeneration. Thus, UPS regulators are attracting attention as drug discovery targets. However, there is still much unknown about the UPS. Our goal is to elucidate the fundamental mechanisms of ubiquitin signaling and proteasomal degradation and to integrate this information into pathophysiology to develop therapeutic strategies for UPS-related diseases. To this end, we are currently focusing on the following research projects.
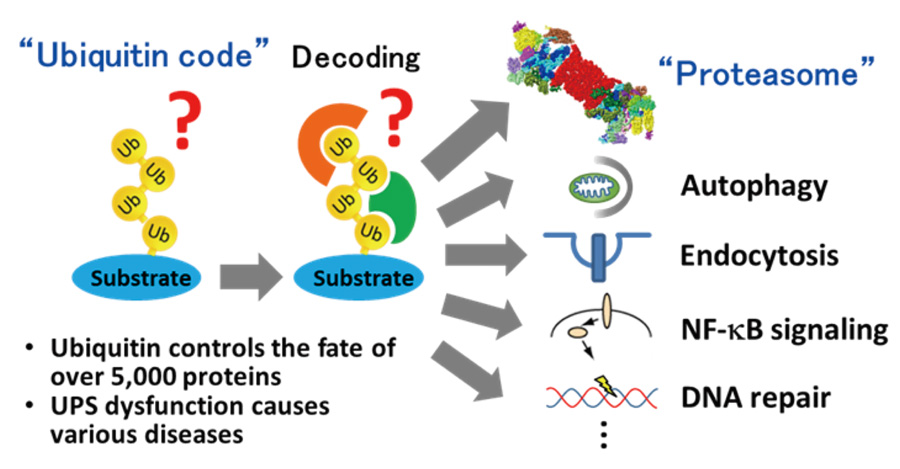
- Deciphering the ubiquitin code: The structural diversity of ubiquitin chains with distinct topologies, called the ‘ubiquitin code,’ regulates the diverse functions of ubiquitin. We have shown that the branching and length of ubiquitin chains provide additional specificity to this code (Nat Commun 2018, Mol Cell 2021). To further investigate the ubiquitin code, we are developing methods to analyze the high-order structure of ubiquitin chains using advanced mass spectrometry.
- Decoding mechanisms for proteasomal degradation: We have identified the p97-UFD1-NPL4 complex and RAD23 family as ubiquitin decoders that direct substrates to the proteasome (Mol Cell 2017, Nat Commun 2019). Currently we are investigating the substrate selectivity of these ubiquitin decoders using advanced proteomics and by developing chemical tools to manipulate proteasomal degradation.
- Biological significance of proteasome phase separation: Recently, we found the ubiquitin-dependent liquid-liquid phase separation (LLPS) of the proteasome under hyperosmotic stress (Nature 2020). This compartmentalization appears to be advantageous for the rapid removal of stress-damaged proteins, and we are further investigating proteasome phase separation under various stress conditions.
- Generation of proteasome mutant mice: Recently, gene mutations in the proteasome have been identified in patients with autism and immune disorders. To understand the pathophysiological mechanism of "proteasomopathy", we generated proteasome mutant mice and are analyzing their phenotypes.
Selected Publications
- Kaiho-Soma A, et al. (2021) "TRIP12 promotes small molecule-induced degradation through K29/K48 branched ubiquitin chains." Mol. Cell 81, 1411-1424
- Yasuda S, Tsuchiya H, Kaiho Ai, et al. (2020) “Stress- and ubiquitylation-dependent phase separation of the proteasome.” Nature 578, 296-300.
- Sato Y, Tsuchiya H, et al. (2019) “Structural insights into ubiquitin recognition and Ufd1 interaction of Npl4.” Nat. Commun. 10, 5708.
- Tsuchiya H, et al. (2018) “Ub-ProT reveals global length and composition of protein ubiquitylation in cells.” Nat. Commun. 9, 524.
- Ohtake F, et al. (2018) “K63 ubiquitylation triggers proteasomal degradation by seeding branched chains.” Proc. Natl. Acad. Sci. USA. 115, E1401-E1408.
- Tsuchiya H, et al. (2017) “In vivo ubiquitin linkage-type analysis reveals that the Cdc48-Rad23/Dsk2 axis contributes to K48-linked chain specificity of the proteasome.” Mol. Cell 66, 485-502.

